
Thermodynamics: An Engineering Approach
8th Edition
ISBN: 9780073398174
Author: Yunus A. Cengel Dr., Michael A. Boles
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1.11, Problem 99RP
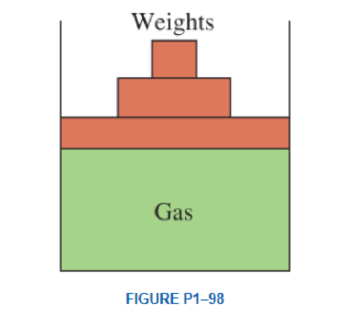
A vertical piston–cylinder device contains a gas at a pressure of 100 kPa. The piston has a mass of 10 kg and a diameter of 14 cm. Pressure of the gas is to be increased by placing some weights on the piston. Determine the local atmospheric pressure and the mass of the weights that will double the pressure of the gas inside the cylinder Answers: 93.6 kPa, 157 kg
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A pump delivering 230 lps of water at 30C has a 300-mm diameter suction pipe and a 254-mm diameter discharge pipe as shown in the figure. The suction pipe is 3.5 m long and the discharge pipe is 23 m long, both pipe's materials are cast iron. The water is delivered 16m above the intake water level. Considering head losses in fittings, valves, and major head loss. a) Find the total dynamic head which the pump must supply. b)It the pump mechanical efficiency is 68%, and the motor efficiency is 90%, determine the power rating of the motor in hp.
The tensile 0.2 percent offset yield strength of AISI 1137 cold-drawn steel bars up to 1 inch in diameter from 2 mills and 25 heats is
reported as follows:
Sy 93
95
101
f
97 99
107 109 111
19 25 38 17 12 10 5 4
103
105
4
2
where Sy is the class midpoint in kpsi and fis the number in each class.
Presuming the distribution is normal, determine the yield strength exceeded by 99.0% of the population.
The yield strength exceeded by 99.0% of the population is
kpsi.
Solve this problem and show all of the work
Chapter 1 Solutions
Thermodynamics: An Engineering Approach
Ch. 1.11 - What is the difference between the classical and...Ch. 1.11 - The value of the gravitational acceleration g...Ch. 1.11 - One of the most amusing things a person can...Ch. 1.11 - An office worker claims that a cup of cold coffee...Ch. 1.11 - 1–5C What is the difference between kg-mass and...Ch. 1.11 - Explain why the light-year has the dimension of...Ch. 1.11 - What is the net force acting on a car cruising at...Ch. 1.11 - 1–8 At 45° latitude, the gravitational...Ch. 1.11 - What is the weight, in N, of an object with a mass...Ch. 1.11 - A 3-kg plastic tank that has a volume of 0.2 m3 is...
Ch. 1.11 - Prob. 11PCh. 1.11 - Prob. 12PCh. 1.11 - Solve Prob. 113 using appropriate software. Print...Ch. 1.11 - A 4-kW resistance heater in a water heater runs...Ch. 1.11 - A 150-lbm astronaut took his bathroom scale (a...Ch. 1.11 - The gas tank of a car is filled with a nozzle that...Ch. 1.11 - Prob. 17PCh. 1.11 - A large fraction of the thermal energy generated...Ch. 1.11 - Prob. 19PCh. 1.11 - 1–20C A can or soft drink at room temperature is...Ch. 1.11 - What is the difference between intensive and...Ch. 1.11 - Is the number of moles of a substance contained in...Ch. 1.11 - Is the state of the air in an isolated room...Ch. 1.11 - The specific weight of a system is defined as the...Ch. 1.11 - What is a quasi-equilibrium process? What is its...Ch. 1.11 - Define the isothermal, isobaric, and isochoric...Ch. 1.11 - Prob. 27PCh. 1.11 - Prob. 28PCh. 1.11 - 1–29C What is specific gravity? How is it related...Ch. 1.11 - 1–31C What are the ordinary and absolute...Ch. 1.11 - Prob. 32PCh. 1.11 - Prob. 33PCh. 1.11 - Prob. 34PCh. 1.11 - Prob. 35PCh. 1.11 - Prob. 36PCh. 1.11 - Prob. 37PCh. 1.11 - Prob. 38PCh. 1.11 - The temperature of a system drops by 45F during a...Ch. 1.11 - Explain why some people experience nose bleeding...Ch. 1.11 - A health magazine reported that physicians...Ch. 1.11 - Someone claims that the absolute pressure in a...Ch. 1.11 - 1–43C Express Pascal’s law, and give a real-world...Ch. 1.11 - Consider two identical fans, one at sea level and...Ch. 1.11 - A vacuum gage connected to a chambee reads 35 kPa...Ch. 1.11 - Prob. 46PCh. 1.11 - 1–47E The pressure in a water line is 1500 kPa....Ch. 1.11 - 1–48E If the pressure inside a rubber balloon is...Ch. 1.11 - A manometer is used to measure the air pressure in...Ch. 1.11 - 1–50 The water in a tank is pressurized by air,...Ch. 1.11 - 1–51 Determine the atmospheric pressure at a...Ch. 1.11 - A 200-pound man has a total foot imprint area of...Ch. 1.11 - The gage pressure in a liquid at a depth of 3 m is...Ch. 1.11 - The absolute pressure in water at a depth of 9 m...Ch. 1.11 - 1–55E Determine the pressure exerted on the...Ch. 1.11 - 1–56 Consider a 70-kg woman who has a total foot...Ch. 1.11 - Prob. 57PCh. 1.11 - The barometer of a mountain hiker reads 750 mbars...Ch. 1.11 - The basic barometer can be used to measure the...Ch. 1.11 - Prob. 61PCh. 1.11 - A gas is contained in a vertical, frictionless...Ch. 1.11 - Reconsider Prob. 158. Using appropriate software,...Ch. 1.11 - Both a gage and a manometer are attached to a gas...Ch. 1.11 - Reconsider Prob. 161. Using appropriate software,...Ch. 1.11 - A manometer containing oil ( = 850 kg/m3) is...Ch. 1.11 - A mercury manometer ( = 13.600 kg/m3) is connected...Ch. 1.11 - Repeat Prob. 165 for a differential mercury height...Ch. 1.11 - The pressure in a natural gas pipeline is measured...Ch. 1.11 - Repeat Prob. 167E by replacing air with oil with a...Ch. 1.11 - Blood pressure is usually measure by wrapping a...Ch. 1.11 - The maximum blood pressure in the upper arm of a...Ch. 1.11 - Prob. 73PCh. 1.11 - Consider a U-tube whose arms are open to the...Ch. 1.11 - Consider a double-fluid manometer attached to an...Ch. 1.11 - Prob. 76PCh. 1.11 - Prob. 77PCh. 1.11 - Calculate the absolute pressure. P1, of the...Ch. 1.11 - Consider the manometer in Fig. 173. If the...Ch. 1.11 - Consider the manometer in Fig. 173. If the...Ch. 1.11 - Consider the system shown in Fig. 177. If a change...Ch. 1.11 - What is the value of the engineering software...Ch. 1.11 - Determine a positive real root of this equation...Ch. 1.11 - Solve this system of three equations with three...Ch. 1.11 - Solve this system of three equations with three...Ch. 1.11 - The reactive force developed by a jet engine to...Ch. 1.11 - A man goes to a traditional market to buy a steak...Ch. 1.11 - What is the weight of a 1-kg substance in N, kN,...Ch. 1.11 - A hydraulic lift is to be used to lift a 1900-kg...Ch. 1.11 - Prob. 92RPCh. 1.11 - Prob. 93RPCh. 1.11 - Prob. 94RPCh. 1.11 - Prob. 95RPCh. 1.11 - Prob. 96RPCh. 1.11 - It is well known that cold air feels much colder...Ch. 1.11 - Reconsider Prob. 1116E. Using appropriate...Ch. 1.11 - A vertical pistoncylinder device contains a gas at...Ch. 1.11 - An air-conditioning system requires a 35-m-long...Ch. 1.11 - The average body temperature of a person rises by...Ch. 1.11 - Balloons are often filled with helium gas because...Ch. 1.11 - Reconsider Prob. 1101. Using appropriate software,...Ch. 1.11 - Determine the maximum amount of load, in kg, the...Ch. 1.11 - The lower half of a 6-m-high cylindrical container...Ch. 1.11 - A vertical, frictionless pistoncylinder device...Ch. 1.11 - A pressure cooker cooks a lot faster than an...Ch. 1.11 - Prob. 108RPCh. 1.11 - Consider a U-tube whose arms are open to the...Ch. 1.11 - Prob. 110RPCh. 1.11 - A water pipe is connected to a double-U manometer...Ch. 1.11 - A gasoline line is connected to a pressure gage...Ch. 1.11 - Repeat Prob. 1110 for a pressure gage reading of...Ch. 1.11 - The average atmosphere pressure on earth is...Ch. 1.11 - Prob. 115RPCh. 1.11 - Prob. 116RPCh. 1.11 - Consider the flow of air through a wind turbine...Ch. 1.11 - The drag force exerted on a car by air depends on...Ch. 1.11 - An apple loses 3.6 kJ of heat as it cools per C...Ch. 1.11 - Consider a fish swimming 5 m below the free...Ch. 1.11 - The atmospheric pressures at the top and the...Ch. 1.11 - Consider a 2.5-m-deep swimming pool. The pressure...Ch. 1.11 - During a heating process, the temperature of an...Ch. 1.11 - At sea level, the weight of 1 kg mass in SI units...
Additional Engineering Textbook Solutions
Find more solutions based on key concepts
Which of the following are illegal variable names in Python, and why? x 99bottles july2009 theSalesFigureForFis...
Starting Out with Python (4th Edition)
CONCEPT QUESTIONS
15.CQ3 The ball rolls without slipping on the fixed surface as shown. What is the direction ...
Vector Mechanics for Engineers: Statics and Dynamics
Using your text editor, enter (that is, type in) the C++ program shown in Display 1.8. Be certain to type the f...
Problem Solving with C++ (10th Edition)
This optional Google account security feature sends you a message with a code that you must enter, in addition ...
SURVEY OF OPERATING SYSTEMS
How is the hydrodynamic entry length defined for flow in a pipe? Is the entry length longer in laminar or turbu...
Fluid Mechanics: Fundamentals and Applications
Comprehension Check 7-14
The power absorbed by a resistor can be given by P = I2R, where P is power in units of...
Thinking Like an Engineer: An Active Learning Approach (4th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- I tried to go through this problem but I don't know what I'm doing wrong can you help me?arrow_forwardGenerate the kinematic diagram of the following mechanisms using the given symbols. Then, draw their graphs and calculate their degrees of freedom (DoF) using Gruebler's formula. PUNTO 2. PUNTO 3. !!!arrow_forwardCreate a schematic representation of the following mechanisms using the given symbols and draw their graphs. Then, calculate their degrees of freedom (DoF) using Gruebler's formula. PUNTO 6. PUNTO 7.arrow_forward
- how the kinematic diagram of the following mechanisms would be represented using the given symbols? PUNTO 0. PUNTO 1. °arrow_forwardCreate a schematic representation of the following mechanisms using the given symbols and draw their graphs. Then, calculate their degrees of freedom (DOF) using Gruebler's formula. PUNTO 4. PUNTO 5. (0) Groundarrow_forwardDraw the graph of ALL the mechanisms and calculate their DoF using Gruebler's formula. PUNTO 0. PUNTO 1.arrow_forward
- An adjustable support. Construction designed to carry vertical load and is adjusted by moving the blue attachment vertically. The link is articulated at both ends (free to rotate) and can therefore only transmit power axially. Analytically calculate the force to which the link is subjected? Calculate analytically rated voltage in the middle of the link.? F=20kN Alpha 30 deg Rel 225 Mpans:5arrow_forwardA swivel crane where the load is moved axially along the beam through the wagon to which the hook is attached. Round bar with a diameter of ∅30 mm. The support beam is articulated at both ends (free to rotate) and can therefore only transmit force axially. Calculate reaction force in the x-direction at point A? Calculate analytical reaction force in the y-direction of point A? Calculate nominal stress in the middle of the support beam?Lengt 5 mAlfa 25 degX=1.5mIPE300-steelmass:1000 kgarrow_forwardgot wrong answers help pleasearrow_forward
- A crate weighs 530 lb and is hung by three ropes attached to a steel ring at A such that the top surface is parallel to the xy plane. Point A is located at a height of h = 42 in above the top of the crate directly over the geometric center of the top surface. Use the dimensions given in the table below to determine the tension in each of the three ropes. 2013 Michael Swanbom cc00 BY NC SA ↑ Z C b B У a D Values for dimensions on the figure are given in the following table. Note the figure may not be to scale. Variable Value a 30 in b 43 in 4.5 in The tension in rope AB is 383 x lb The tension in rope AC is 156 x lb The tension in rope AD is 156 x lbarrow_forwardA block of mass m hangs from the end of bar AB that is 7.2 meters long and connected to the wall in the xz plane. The bar is supported at A by a ball joint such that it carries only a compressive force along its axis. The bar is supported at end B by cables BD and BC that connect to the xz plane at points C and D respectively with coordinates given in the figure. Cable BD is elastic and can be modeled as a linear spring with a spring constant k = 400 N/m and unstretched length of 6.34 meters. Determine the mass m, the compressive force in beam AB and the tension force in cable BC. Z C D (c, 0, d) (a, 0, b) A B y f m cc 10 BY NC SA 2016 Eric Davishahl x Values for dimensions on the figure are given in the following table. Note the figure may not be to scale. Variable Value a 8.1 m b 3.3 m с 2.7 m d 3.9 m e 2 m f 5.4 m The mass of the block is 68.8 The compressive force in bar AB is 364 × kg. × N. The tension in cable BC is 393 × N.arrow_forwardThe airplane weighs 144100 lbs and flies at constant speed and trajectory given by 0 on the figure. The plane experiences a drag force of 73620 lbs. 0 a.) If 11.3°, determine the thrust and lift forces = required to maintain this speed and trajectory. b.) Next consider the case where is unknown, but it is known that the lift force is equal to 7.8 times the quantity (Fthrust Fdrag). Compute the resulting trajectory angle and the lift force in this case. Use the same values for the weight and drag forces as you used for part a. 20. YAAY' Farag Ө Fthrust CC + BY NC SA 2013 Michael Swanbom Flift Fweight The lift force acts in the y' direction. The weight acts in the negative y direction. The thrust and drag forces act in the positive and negative x' directions respectively. Part (a) The thrust force is equal to 101,855 ☑ lbs. The lift force is equal to 141,282 ☑ lbs. Part (b) The trajectory angle 0 is equal to 7.31 ✓ deg. The lift force is equal to 143,005 ☑ lbs.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Refrigeration and Air Conditioning Technology (Mi...Mechanical EngineeringISBN:9781305578296Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill JohnsonPublisher:Cengage Learning
Refrigeration and Air Conditioning Technology (Mi...Mechanical EngineeringISBN:9781305578296Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill JohnsonPublisher:Cengage Learning

Refrigeration and Air Conditioning Technology (Mi...
Mechanical Engineering
ISBN:9781305578296
Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:Cengage Learning
Thermodynamics: Maxwell relations proofs 1 (from ; Author: lseinjr1;https://www.youtube.com/watch?v=MNusZ2C3VFw;License: Standard Youtube License