
Concept explainers
Interpretation:
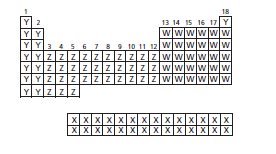
The subshell in which the valence electrons of elements labelled as W are present needs to be determined.
Concept introduction:
A tabular arrangement of the chemical elements by their
Answer to Problem 8STP
The correct option is (b), p.
Explanation of Solution
Reason for correct option:
The electrons present in the outermost orbital of highest principal energy level are said to be valence electrons. The possible orbitals are
In periodic table, the elements are arranged in increasing atomic number and the elements in the same group have similar chemical and physical properties. The valence electrons of group 1 and 2 elements is in
The elements labeled W are present in group 13-18 so, the valence electrons are present in
Reason for correct options:
The elements labeled Y are present in group 1-2 so, the valence electrons are present in
The elements labeled Z are present in group 3-12 so, the valence electrons are present in
The elements labeled X have the valence electrons in
Chapter 11 Solutions
Glencoe Chemistry: Matter and Change, Student Edition
Additional Science Textbook Solutions
Applications and Investigations in Earth Science (9th Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
Chemistry: The Central Science (14th Edition)
Introductory Chemistry (6th Edition)
Campbell Biology: Concepts & Connections (9th Edition)
Human Physiology: An Integrated Approach (8th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





