
Interpretation:
The molecular shape should be selected for a given molecule that have four shared pair of electrons and no lone pairs of electrons from the given shapes.
Concept introduction:
The geometric shape of the molecule is predicted by VSEPR theory (Valence shell electron pair repulsion theory) from electron pairs surrounding the central atom of the molecule whereas the molecular shape of the molecule is predicted by number of electron pairs as well as lone pairs.
Answer to Problem 14STP
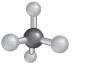
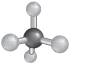
The correct option is (C):
Explanation of Solution
Given:
Reason for correct option:
The molecular shape can be determined for a molecule that have four shared pair of electrons and no lone pairs of electrons by taking the example of alkane.
- The structure of
alkanes has single bond between carbon and hydrogen atoms. So, in alkane there is 4 bonded atoms and no lone pair across the carbon atom and thus, according to VESPR the shape of the molecule will be tetrahedral.
Hence, the correct option is (C):
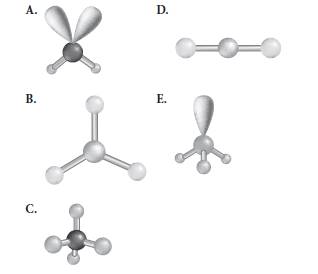
Reason for correct options:
The geometry A and E have 2 and 1 lone pair respectively whereas geometry B and D both possess less than 4 number of electron pairs.
Chapter 11 Solutions
Glencoe Chemistry: Matter and Change, Student Edition
Additional Science Textbook Solutions
Campbell Biology: Concepts & Connections (9th Edition)
College Physics: A Strategic Approach (3rd Edition)
Biology: Life on Earth (11th Edition)
Cosmic Perspective Fundamentals
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Campbell Biology (11th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





