
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 11, Problem 53P
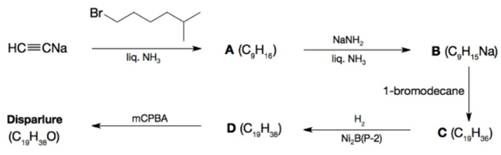
Outlined below is a synthesis of the gypsy moth sex attractant disparlure (a pheromone). Give the structure of disparlure and intermediates A-D.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
solve please
Please answer the question and provide a detailed drawing of the structure. If there will not be a new C – C bond, then the box under the drawing area will be checked.
Will the following reaction make a molecule with a new C – C bond as its major product:
Draw the major organic product or products, if the reaction will work. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry.
Please do not use AI. AI cannot "see" the molecules properly, and it therefore gives the wrong answer while giving incorrect descriptions of the visual images we're looking at. All of these compounds would be produced (I think). In my book, I don't see any rules about yield in this case, like explaining that one product would be present in less yield for this reason or that reason. Please explain why some of these produce less yield than others.
Chapter 11 Solutions
Organic Chemistry
Ch. 11 - Prob. 1PPCh. 11 - Practice Problem 11.2 Give bond-line formulas and...Ch. 11 - Prob. 3PPCh. 11 - Prob. 4PPCh. 11 - Prob. 5PPCh. 11 - Prob. 6PPCh. 11 - Prob. 7PPCh. 11 - Practice Problem 11.8 Show how you would prepare...Ch. 11 - Prob. 9PPCh. 11 - Prob. 10PP
Ch. 11 - Practice Problem 11.11
An exception to what is...Ch. 11 - Prob. 12PPCh. 11 - Prob. 13PPCh. 11 - Prob. 14PPCh. 11 - Prob. 15PPCh. 11 - Prob. 16PPCh. 11 - Prob. 17PPCh. 11 - Prob. 18PPCh. 11 - Practice Problem 11.19
Propose structures for each...Ch. 11 - Prob. 20PPCh. 11 - Prob. 21PPCh. 11 - Prob. 22PPCh. 11 - Prob. 23PPCh. 11 - Prob. 24PPCh. 11 - Give an IUPAC substitutive name for each of the...Ch. 11 - Prob. 26PCh. 11 - Prob. 27PCh. 11 - Prob. 28PCh. 11 - Prob. 29PCh. 11 - 11.30. Show how you might prepare 2-bromobutane...Ch. 11 - Prob. 31PCh. 11 - Prob. 32PCh. 11 - Prob. 33PCh. 11 - Considering A-L to represent the major products...Ch. 11 - Write structures for the products that would be...Ch. 11 - Prob. 36PCh. 11 - Provide the reagents necessary for the following...Ch. 11 - 11.38. Predict the major product from each of the...Ch. 11 - Predict the products from each of the following...Ch. 11 - Provide the reagents necessary to accomplish the...Ch. 11 - 11.41. Provide reagents that would accomplish the...Ch. 11 - Prob. 42PCh. 11 - 11.43. A synthesis of the -receptor blocker called...Ch. 11 - Prob. 44PCh. 11 - Prob. 45PCh. 11 - 11.46. For each of the following, write a...Ch. 11 - 11.47. Vicinal halo alcohols (halohydrins) can be...Ch. 11 - Prob. 48PCh. 11 - Prob. 49PCh. 11 - Prob. 50PCh. 11 - Prob. 51PCh. 11 - Prob. 52PCh. 11 - Outlined below is a synthesis of the gypsy moth...Ch. 11 - Prob. 54PCh. 11 - Prob. 55PCh. 11 - Prob. 56PCh. 11 - 11.57. When the 3-bromo-2-butanol with the...Ch. 11 - 11.58. Reaction of an alcohol with thionyl...Ch. 11 - Prob. 59PCh. 11 - Prob. 60PCh. 11 - Prob. 1LGPCh. 11 - Prob. 2LGPCh. 11 - Synthesize the compound shown below from...
Additional Science Textbook Solutions
Find more solutions based on key concepts
How can 1H NMR distinguish between the compounds in each of the following pairs?
Organic Chemistry (8th Edition)
41. Humans vary in many ways from one another. Among many minor phenotypic differences are the following five i...
Genetic Analysis: An Integrated Approach (3rd Edition)
Use the following graph to answer questions 3 and 4. 3. Which of the lines best depicts the log phase of a ther...
Microbiology: An Introduction
Fibrous connective tissue consists of ground substance and fibers that provide strength, support, and flexibili...
Human Biology: Concepts and Current Issues (8th Edition)
6.1 State the number of electrons that be must be lost by atoms of each of the following to achieve a stable el...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
The portion of 1.0 L of 0.50 M blue dye solution having the same number of moles as 1.0 L of 0.25 M blue dye so...
Living By Chemistry: First Edition Textbook
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please answer the question and provide detailed explanations.arrow_forwardAll of these compounds would be produced (I think). In my book, I don't see any rules about yield in this case, like explaining that one product would be present in less yield for this reason or that reason. Please explain why some of these produce less yield than others.arrow_forward5. Fill in the missing molecules in the following reaction pathway. TMSO Heat + CI then HF O₂N (1.0 equiv) AICI 3 OMearrow_forward
- e. O₂N NO2 1. excess H2, Pd/C 2. excess NaNO2, HCI 3. excess CuCNarrow_forwardHelp with a periodic table task.' Procedure Part 1: Customizing a Periodic Table Use a textbook or other valid source to determine which elements are metals, nonmetals, metalloids (called semimetals in some texts), alkali metals, alkaline earth metals, transition metals, halogens, and noble gases. Download and print a copy of the Periodic Table of Elements. Use colored pencils, colorful highlighters, or computer drawing tools to devise a schematic for designating each of the following on the periodic table: Group numbers Period number Labels for these groups: alkali metals, alkaline earth metals, transition metals, inner transition metals (lanthanides and actinides), other metals, metalloids (semimetals), other nonmetals, halogens, and noble gases Metals, nonmetals, and metalloids Note: Write the group and period numbers and color/highlight each element for categorization. Be sure to include a key for the schematic. Take a photo of the completed periodic table and upload the…arrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
- Can you explain these two problems for mearrow_forward个 ^ Blackboard x Organic Chemistry II Lecture (m x Aktiv Learning App x → C app.aktiv.com ← Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Problem 28 of 35 :OH H HH KO Select to Edit Arrows CH CH₂OK, CH CH2OH 5+ H :0: Donearrow_forwardCan you explain those two problems for me please.arrow_forward
- Do we need to draw the "ethyne" first for this problem? im confusedarrow_forwardCan you explain how this problem was solved.arrow_forwardQuestion 2 show work. don't Compound give Ai generated solution So (J K-1 mol-1) A 26 B 54 C 39 D 49 At 298 K, AG° is 375 kJ for the reaction 1A + 1B → 4C + 2D Calculate AH° for this reaction in kJ.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Characteristic Reactions of Benzene and Phenols; Author: Linda Hanson;https://www.youtube.com/watch?v=tjEqEjDd87E;License: Standard YouTube License, CC-BY
An Overview of Aldehydes and Ketones: Crash Course Organic Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=-fBPX-4kFlw;License: Standard Youtube License