
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 11, Problem 53E
For each of the following pairs, predict which substance would be more soluble in water.
a.  or
or 
b. 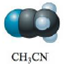 or
or 
c. 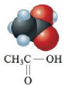 or
or 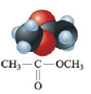
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
11. Complete the following esterification reaction with names of all the reactants and products under.
Hint: Remove the water and end up with ester
R-C-OH + ROH
R-C-OR + H₂O
A carboxylic acid
An alcohol
An ester
Water
BYJU'S
H-C-C
O-H
Нин
C-C-C-H
HAAA
H O-C-C-C-H
AAA
Ethanoic acid
Propanol
Water Propyl ethanoate
By com
CH3COOH + CH3CH2CH2CH₂CH₂OH →
Practice for alcohols aldehydes and ketones:
12. Draw the structures from the following names mixed of alcohol/aldehyde and ketone:
a. 4-methyl cyclohexanone
b. 3-methyl-2-pentenal
c. 2,3-dimethylcyclohexanone
d. 1,3propanediol or Propane 1,3 diol
13. Write systematic names for the following compounds identify
functional group:
a.
b. (CH3)2CH-C
OH
c) CH(CH₂)--
OH
-,-,
may you please show all steps! i am having a hard time understanding and applying in this format, thank you!
10. Complete the substitution reaction of 2 pentanol with these reagents.
Reagents & Reaction Conditions use practice sheet. Please write only
major products, minor product like water, other gases are not
required.
Hint: In substitution of alcohol, we generally substitute OH group
with Halogens like cl, Br, F using some reagent containing
halogens. Ensure to add halogens to the same carbon number
where you are removing OH from
Examples
Alcohols can be converted to Alkyl Halides with HX acids
HBr
H₂O
HCI
+ H₂O
HI
+
H₂O
CH,CH₂OH + SOCI₂
CH,CH₂OH + PCI₁₂
A
BBYJU'S
CH CHCI + SO₂+ HCI
CH₂CH CIP(OH), + HCI
CH,CH₂OH + PCI CHCHCI + POCI + HCI
CH,CH₂OH + PBr, CH,CH,Br + P(OH), + HBr
1. Reaction with HBr with 2 Pentanol
2.Reaction with HI with 2 pentanol
© Byjus.com
3.Reaction with HCI+ZnCl,, with 2 pentanol (Zncl2 is catalyst no role)
4.Reaction with SOCI,, with 2 Pentanol
5.Reaction with PBr; or PCl, with 2 pentanol
Chapter 11 Solutions
Chemistry
Ch. 11 - Prob. 1RQCh. 11 - Using KF as an example, write equations that refer...Ch. 11 - Prob. 3RQCh. 11 - Prob. 4RQCh. 11 - Define the terms in Raoults law. Figure 10-9...Ch. 11 - In terms of Raoults law, distinguish between an...Ch. 11 - Vapor-pressure lowering is a colligative property,...Ch. 11 - What is osmotic pressure? How is osmotic pressure...Ch. 11 - Distinguish between a strong electrolyte, a weak...Ch. 11 - Prob. 10RQ
Ch. 11 - Prob. 1ALQCh. 11 - Once again, consider Fig. 10-9. Suppose instead of...Ch. 11 - Prob. 3ALQCh. 11 - Prob. 4ALQCh. 11 - You have read that adding a solute to a solvent...Ch. 11 - You drop an ice cube (made from pure water) into a...Ch. 11 - Using the phase diagram for water and Raoults law,...Ch. 11 - You and your friend are each drinking cola from...Ch. 11 - Prob. 9ALQCh. 11 - Prob. 10ALQCh. 11 - If a solution shows positive deviations from...Ch. 11 - Prob. 12ALQCh. 11 - Rubbing alcohol contains 585 g isopropanol...Ch. 11 - Prob. 14SRCh. 11 - Prob. 15SRCh. 11 - Prob. 16SRCh. 11 - Calculate the sodium ion concentration when 70.0...Ch. 11 - Write equations showing the ions present after the...Ch. 11 - Rationalize the temperature dependence of the...Ch. 11 - The weak electrolyte NH3(g) does not obey Henrys...Ch. 11 - The two beakers in the sealed container...Ch. 11 - The following plot shows the vapor pressure of...Ch. 11 - When pure methanol is mixed with water, the...Ch. 11 - Prob. 24QCh. 11 - For an acid or a base, when is the normality of a...Ch. 11 - Prob. 26QCh. 11 - Prob. 27QCh. 11 - Prob. 28QCh. 11 - Prob. 29QCh. 11 - Table sugar (C12H22O11) or urea [(NH2)2CO] can be...Ch. 11 - If two different aqueous solutions of proteins...Ch. 11 - An extremely important application of dialysis is...Ch. 11 - Explain the terms isotonic solution, crenation,...Ch. 11 - Prob. 34QCh. 11 - Prob. 35ECh. 11 - A typical IV used in hospitals is dextrose 5% in...Ch. 11 - Prob. 37ECh. 11 - Prob. 38ECh. 11 - Common commercial acids and bases are aqueous...Ch. 11 - In lab you need to prepare at least 100 mL of each...Ch. 11 - Prob. 41ECh. 11 - Prob. 42ECh. 11 - Prob. 43ECh. 11 - Calculate the molarity and mole fraction of...Ch. 11 - Prob. 45ECh. 11 - Prob. 46ECh. 11 - Prob. 47ECh. 11 - a. Use the following data to calculate the...Ch. 11 - Although Al(OH)3 is insoluble in water, NaOH is...Ch. 11 - Prob. 50ECh. 11 - Prob. 51ECh. 11 - Prob. 52ECh. 11 - For each of the following pairs, predict which...Ch. 11 - Which ion in each of the following pairs would you...Ch. 11 - Rationalize the trend in water solubility for the...Ch. 11 - In flushing and cleaning columns used in liquid...Ch. 11 - The solubility of nitrogen in water is 8.21 104...Ch. 11 - Calculate the solubility of O2 in water at a...Ch. 11 - The vapor pressure of a solution containing 53.6 g...Ch. 11 - An aqueous solution containing glucose has a vapor...Ch. 11 - The normal boiling point of diethyl ether is...Ch. 11 - At a certain temperature, the vapor pressure of...Ch. 11 - Prob. 63ECh. 11 - A solution is prepared by mixing 0.0300 mole of...Ch. 11 - What is the composition of a methanol...Ch. 11 - Benzene and toluene form an ideal solution....Ch. 11 - Which of the following will have the lowest total...Ch. 11 - Prob. 68ECh. 11 - Match the vapor pressure diagrams with the...Ch. 11 - The vapor pressures of several solutions of...Ch. 11 - A solution is prepared by dissolving 27.0 g urea,...Ch. 11 - A 2.00-g sample of a large biomolecule was...Ch. 11 - What mass of glycerin (C3H8O3), a nonelectrolyte,...Ch. 11 - The freezing point of 1-butanol is 25.50C and Kf...Ch. 11 - Prob. 75ECh. 11 - What volume of ethylene glycol (C2H6O2), a...Ch. 11 - Reserpine is a natural product isolated from the...Ch. 11 - A solution contains 3.75 g of a nonvolatile pure...Ch. 11 - a. Calculate the freezing-point depression and...Ch. 11 - Erythrocytes are red blood cells containing...Ch. 11 - Prob. 81ECh. 11 - Prob. 82ECh. 11 - Prob. 83ECh. 11 - Prob. 84ECh. 11 - Consider the following solutions: 0.010 m Na3PO4...Ch. 11 - From the following: pure water solution of...Ch. 11 - Prob. 87ECh. 11 - Prob. 88ECh. 11 - Prob. 89ECh. 11 - Consider the following representations of an ionic...Ch. 11 - Prob. 91ECh. 11 - Prob. 92ECh. 11 - Use the following data for three aqueous solutions...Ch. 11 - The freezing-point depression of a 0.091-m...Ch. 11 - Prob. 95ECh. 11 - A 0.500-g sample of a compound is dissolved in...Ch. 11 - The solubility of benzoic acid (HC7H5O2), is 0.34...Ch. 11 - Prob. 99AECh. 11 - Prob. 100AECh. 11 - Prob. 101AECh. 11 - In Exercise 96 in Chapter 8, the pressure of CO2...Ch. 11 - Explain the following on the basis of the behavior...Ch. 11 - The term proof is defined as twice the percent by...Ch. 11 - Prob. 105AECh. 11 - Prob. 106AECh. 11 - A solution is made by mixing 50.0 g acetone...Ch. 11 - Prob. 108AECh. 11 - Thyroxine, an important hormone that controls the...Ch. 11 - Prob. 110AECh. 11 - An unknown compound contains only carbon,...Ch. 11 - Prob. 112AECh. 11 - Prob. 113AECh. 11 - Prob. 115AECh. 11 - Patients undergoing an upper gastrointestinal...Ch. 11 - Prob. 118CWPCh. 11 - The lattice energy of NaCl is 786 kJ/mol, and the...Ch. 11 - For each of the following pairs, predict which...Ch. 11 - The normal boiling point of methanol is 64.7C. A...Ch. 11 - A solution is prepared by mixing 1.000 mole of...Ch. 11 - Prob. 123CWPCh. 11 - A 4.7 102 mg sample of a protein is dissolved in...Ch. 11 - A solid consists of a mixture of NaNO3 and...Ch. 11 - The vapor pressure of pure benzene is 750.0 torr...Ch. 11 - Prob. 127CPCh. 11 - Plants that thrive in salt water must have...Ch. 11 - You make 20.0 g of a sucrose (C12H22O11) and NaCl...Ch. 11 - Prob. 130CPCh. 11 - The vapor in equilibrium with a pentane-hexane...Ch. 11 - A forensic chemist is given a white solid that is...Ch. 11 - A 1.60-g sample of a mixture of naphthalene...Ch. 11 - Prob. 134CPCh. 11 - Prob. 135CPCh. 11 - You have a solution of two volatile liquids, A and...Ch. 11 - In some regions of the southwest United States,...Ch. 11 - Specifications for lactated Ringers solution,...Ch. 11 - Creatinine, C4H7N3O, is a by-product of muscle...Ch. 11 - An aqueous solution containing 0.250 mole of Q, a...Ch. 11 - Anthraquinone contains only carbon, hydrogen, and...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Why are mutants used as test organisms in the Ames test?
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
Single penny tossed 20 times and counting heads and tails: Probability (prediction): _______/20 heads ________/...
Laboratory Manual For Human Anatomy & Physiology
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
How does the removal of hydrogen atoms from nutrient molecules result in a loss of energy from the nutrient mol...
SEELEY'S ANATOMY+PHYSIOLOGY
The validity of a scientific law.
Physical Universe
An obese 55-year-old woman consults her physician about minor chest pains during exercise. Explain the physicia...
Biology: Life on Earth with Physiology (11th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 3. Is 2-methyl-2-propanol a primary, secondary, or tertiary alcohol? Write out the structures of 2-methyl-2-propanol and also any oxidation products of 2- methyl-2- propanol. If there is more than one oxidation product, give the structure of each of the products. 4. 2-Propanol is the IUPAC systematic name of this alcohol. It has a common name by which it is much better known (You'll see it in the grocery store or pharmacy). Give that common name 5. Aldehydes can be synthesized by the oxidation of. Please choose from below choices A. Primary alcohols B. Secondary alcohols C. Organic acids D. Inorganic acids 6. Tertiary alcohol Can undergo oxidation. yes or no. ? If yes then answer the product.arrow_forwardFinish the reactions hand written pleasearrow_forwardPart A Identify each alcohol as primary, secondary, or tertiary Drag the appropriate items to their respective bins. CH₂ H₂C- -C-OH HO CH₂ Primary Он OH CH₂ OH CCH₂OH CH₂ сн Secondary Tertiary Reset Help CH,CH₂ (CH)CHCH,OH CH,CH,CH,CCH, CHOH CH₂ Different types of alcohol groups Alcohol and its reaction: 8. Combing two alcohol molecules below and completing the reaction with Product .( Hint Reaction called etherification as ether is formed and name the ether once you complete the reaction. Hint.: R-O-H+H-O-RR-O-R Do the reaction: CH₂OH + CH₂OH---→ + H-O-H 9. Write the reaction of formation of alcohol from alkene by adding water: Addition reaction also called hydration reaction as we are adding water which occur always in presence of acid Hint: Break the double bond and add H and OH if symmetrical then add anywhere if unsymmetrical then follow Markovnikov rule H should go to that double bone carbon which has more hydrogen CH2=CH2 + H₂O-→arrow_forward
- Complete the reaction hand written pleasearrow_forwardPredict the major products of this organic reaction: HBr (1 equiv) cold ? Some important notes: • Draw the major product, or products, of this reaction in the drawing area below. • You can draw the products in any arrangement you like. • Pay careful attention to the reaction conditions, and only include the major products. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. • Note that there is only 1 equivalent of HBr reactant, so you need not consider the case of multiple additions. dm Re Explanation Check ©2025 McGraw Hill LLC. All Rights Reserved. Termarrow_forwardb) Use curved arrows to show the reaction of the radical with hydrogen bromide. Br: Br H .. Answer Bankarrow_forward
- Indicate the products of the reaction between CH3COCH2COONa (Sodium acetoacetate) and BrCH2COOC2H5arrow_forwardIndicate whether the product of the reaction between Naphthalene and CrO3 in acetic acid at 25ºC is 1,4 naphthoquinone or phthalic anhydride.arrow_forwardIndicate the products of the reaction between CH3COCH2COOC2H5 and Na+-OC2H5.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER
Solutions: Crash Course Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=9h2f1Bjr0p4;License: Standard YouTube License, CC-BY