
Concept explainers
(a)
Interpretation:
The structure of the alkene that could have been used to produce deuterated bromoalkene when treated with deuterium bromide (
Concept introduction:
The electrophilic addition of a Bronsted acid to the carbon-carbon double bond in
Answer to Problem 11.8P
The structure of the corresponding alkene that has been used to produce the given deuterated bromoalkane is
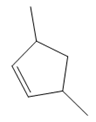
Explanation of Solution
The structure of the given bromoalkane is
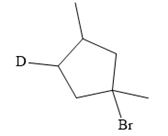
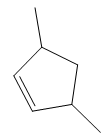
In the structure above, the carbon atom to which the bromine atom is attached must be the carbon bearing the positive charge. Thus, a stable tertiary carbocation must have been formed during the mechanism. Deuterium is an isotope of hydrogen having similar chemical properties. When an alkene is treated with deuterium bromide, the first step of addition is that the deuterium gets attached to the doubly bonded carbon atom which has got a higher number of hydrogens attached (or least substituted). Thus, the carbon atom to which deuterium is attached must be having a double bond in the original alkene. The resulting carbocation that would form will be secondary and can rearrange to a more stable, tertiary carbocation via 1, 2-hydride shift. Thus, a less stable carbocation undergoes carbocation rearrangement reaction to form the most stable carbocation. In the next step, the bromine ion attacks this tertiary carbocation to form the given product. Thus, the structure of the corresponding alkene that could have been used to produce the given deuterated bromoalkane must be
The complete mechanism is shown below:
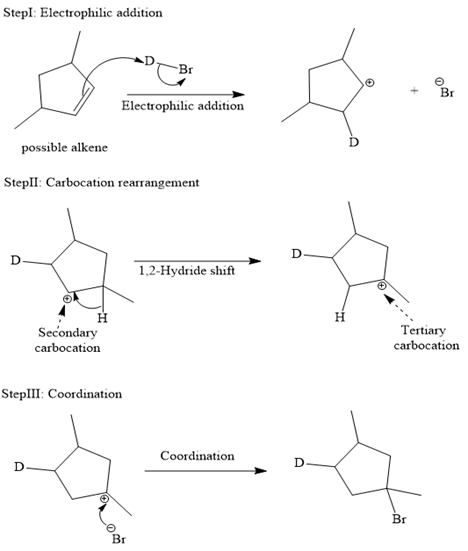
In an electrophilic addition reaction, the carbocation rearrangement reaction takes place to form a rearranged product.
(b)
Interpretation:
The structure of the alkene that could have been used to produce deuterated bromoalkene when treated with deuterium bromide (
Concept introduction:
The electrophilic addition of a Bronsted acid to the carbon-carbon double bond in alkenes is susceptible to carbocation rearrangements due to the stability of the carbocation. The carbocation rearrangement occurs via either 1, 2 hydride shift or 1, 2 methyl shift depending on the stability of the carbocation formed. The stability order for carbocation is benzylic > tertiary > secondary > primary > methyl etc. The normal electrophilic addition gives 1, 2-addition product, but due to the rearrangement reaction, the rearranged product may be formed instead of 1, 2-addition product. In the reaction of an alkene and hydrogen halide or deuterium halide, the carbon to which the deuterium is attached is the carbon bearing the positive charge. Further, the carbon atom to which the halide atom is attached must be the carbon bearing the positive charge, that is, carbocation intermediate. Rearrangements of the carbocation intermediate may be observed if a more stable carbocation is possible.
Answer to Problem 11.8P
The structure of the corresponding alkene that has been used to produce the given deuterated bromoalkane is
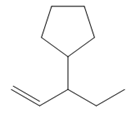
Explanation of Solution
The structure of the given bromoalkane is
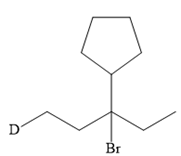
In the structure above, the carbon atom to which the bromine atom is attached must be the carbon bearing the positive charge. Thus, a stable tertiary carbocation must have been formed during the mechanism. Deuterium is an isotope of hydrogen having similar chemical properties. When an alkene is treated with deuterium bromide, the first step of addition is that the deuterium gets attached to the doubly bonded carbon atom which has got a higher number of hydrogens attached (or least substituted). Thus, the carbon atom to which deuterium is attached must be having a double bond in the original alkene. The resulting carbocation that would form will be secondary and can rearrange to a more stable, tertiary carbocation via 1, 2-hydride shift. Thus, a less stable carbocation undergoes carbocation rearrangement reaction to form the most stable carbocation. In the next step, the bromine ion attacks this tertiary carbocation to form the given product.
Thus, the structure of the corresponding alkene that could have been used to produce the given deuterated bromoalkane must be
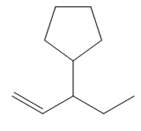
The complete mechanism is shown below:
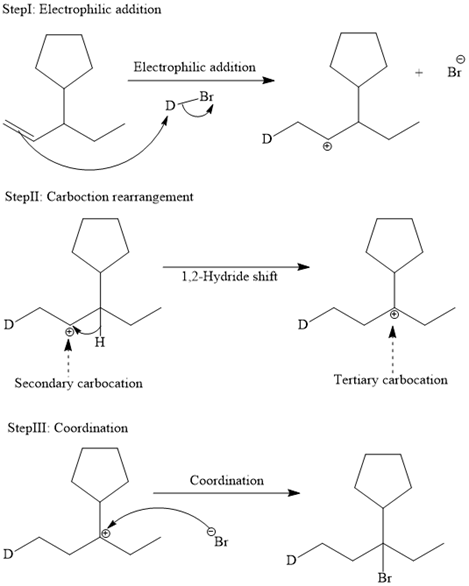
In an electrophilic addition reaction, the carbocation rearrangement reaction takes place to form a rearranged product.
(c)
Interpretation:
The structure of the alkene that could have been used to produce deuterated bromoalkene when treated with deuterium bromide (
Concept introduction:
The electrophilic addition of a Bronsted acid to the carbon-carbon double bond in alkenes is susceptible to carbocation rearrangements due to the stability of the carbocation. The carbocation rearrangement occurs via either 1, 2 hydride shift or 1, 2 methyl shift depending on the stability of the carbocation formed. The stability order for carbocation is benzylic > tertiary > secondary > primary > methyl etc. The normal electrophilic addition gives 1, 2-addition product, but due to the rearrangement reaction, the rearranged product may be formed instead of 1, 2-addition product. In the reaction of an alkene and hydrogen halide or deuterium halide, the carbon to which the deuterium is attached is the carbon bearing the positive charge. Further, the carbon atom to which the halide atom is attached must be the carbon bearing the positive charge, that is, carbocation intermediate. Rearrangements of the carbocation intermediate may be observed if a more stable carbocation is possible.
Answer to Problem 11.8P
The structure of the corresponding alkene that has been used to produce the given deuterated bromoalkane is
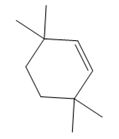
Explanation of Solution
The structure of the given bromoalkane is
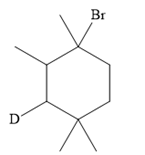
In the structure above, the carbon atom to which the bromine atom is attached must be the carbon bearing the positive charge. Thus, a stable tertiary carbocation must have been formed during the mechanism. Deuterium is an isotope of hydrogen having similar chemical properties. When an alkene is treated with deuterium bromide, the first step of addition is that the deuterium gets attached to the doubly bonded carbon atom, which has got a higher number of hydrogens attached (or least substituted). Thus, the carbon atom to which deuterium is attached must be having a double bond in the original alkene. The resulting carbocation that would form will be secondary and can rearrange to a more stable, tertiary carbocation via 1, 2-hydride shift. Thus, a less stable carbocation undergoes carbocation rearrangement reaction to form the most stable carbocation. In the next step, the bromine ion attacks this tertiary carbocation to form the given product.
Thus, the structure of the corresponding alkene that has been used to produce the given deuterated bromoalkane is
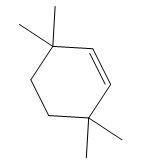
The complete mechanism is shown below:
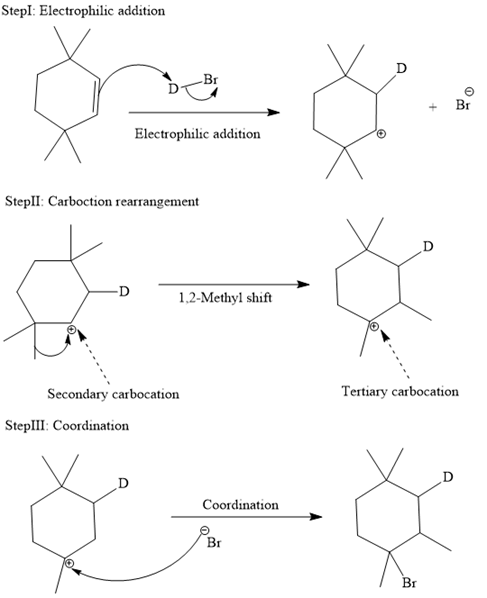
In an electrophilic addition reaction, the carbocation rearrangement reaction takes place to form a rearranged product.
(d)
Interpretation:
The structure of the alkene that could have been used to produce deuterated bromoalkene when treated with deuterium bromide (
Concept introduction:
The electrophilic addition of a Bronsted acid to the carbon-carbon double bond in alkenes is susceptible to carbocation rearrangements due to the stability of the carbocation. The carbocation rearrangement occurs via either 1, 2 hydride shift or 1, 2 methyl shift depending on the stability of the carbocation formed. The stability order for carbocation is benzylic > tertiary > secondary > primary > methyl etc. The normal electrophilic addition gives 1, 2-addition product, but due to the rearrangement reaction, the rearranged product may be formed instead of 1, 2-addition product. In the reaction of an alkene and hydrogen halide or deuterium halide, the carbon to which the deuterium is attached is the carbon bearing the positive charge. Further, the carbon atom to which the halide atom is attached must be the carbon bearing the positive charge, that is, carbocation intermediate. Rearrangements of the carbocation intermediate may be observed if a more stable carbocation is possible.
Answer to Problem 11.8P
The structure of the corresponding alkene that has been used to produce the given deuterated bromoalkane is
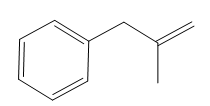
Explanation of Solution
The structure of the given bromoalkane is
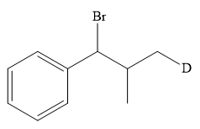
In the structure above, the carbon atom to which the bromine atom is attached must be the carbon bearing the positive charge. Thus, a stable tertiary carbocation must have been formed during the mechanism. Deuterium is an isotope of hydrogen having similar chemical properties. When an alkene is treated with deuterium bromide, the first step of addition is that the deuterium gets attached to the doubly bonded carbon atom, which has got a higher number of hydrogens attached (or least substituted). Thus, the carbon atom to which deuterium is attached must be having a double bond in the original alkene. The resulting carbocation that would form will be secondary and can rearrange to a more stable, tertiary carbocation via 1, 2-hydride shift. Thus, a less stable carbocation undergoes carbocation rearrangement reaction to form the most stable carbocation. In the next step, the bromine ion attacks this tertiary carbocation to form the given product.
Thus, the structure of the corresponding alkene that has been used to produce the given deuterated bromoalkane is
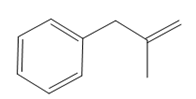
The mechanism for the reaction is shown below:
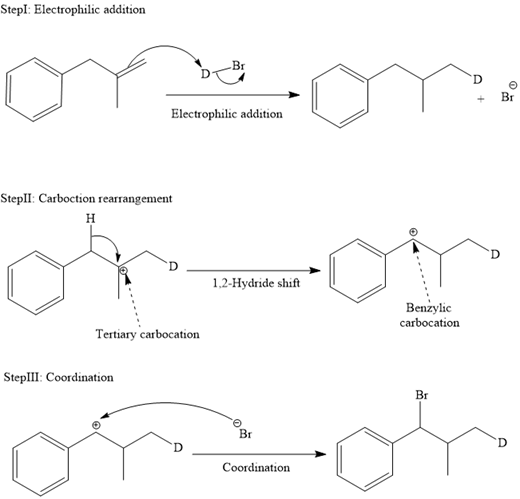
In an electrophilic addition reaction, the carbocation rearrangement reaction takes place to form a rearranged product.
Want to see more full solutions like this?
Chapter 11 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- help 20arrow_forwardProvide the drawing of the unknown structure that corresponds with this data.arrow_forward20.44 The Diels-Alder reaction is not limited to making six-membered rings with only car- bon atoms. Predict the products of the following reactions that produce rings with atoms other than carbon in them. OCCH OCCH H (b) CH C(CH₂)s COOCH མ་ནས་བ (c) N=C H -0.X- (e) H C=N COOCHS + CH2=CHCH₂ →→arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

