
Organic Chemistry
5th Edition
ISBN: 9780078021558
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 11, Problem 11.47P
Draw a stepwise mechanism for each reaction.
a. 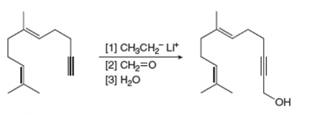
b. 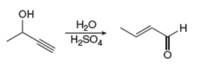
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Please draw the structure in the box that is consistent with all the spectral data and
alphabetically label all of the equivalent protons in the structure (Ha, Hb, Hc....) in order to assign all
the proton NMR peaks. The integrations are computer generated and approximate the number of
equivalent protons. Molecular formula: C13H1802
14
13
12
11
10
11 (ppm)
Structure with assigned H peaks
2.08
3.13
A 0.10 M solution of acetic acid (CH3COOH, Ka = 1.8 x 10^-5) is titrated with a 0.0250 M solution of magnesium hydroxide (Mg(OH)2). If 10.0 mL of the acid solution is titrated with 10.0 mL of the base solution, what is the pH of the resulting solution?
Firefly luciferin exhibits three rings. Identify which of the rings are aromatic. Identify which lone pairs are involved in establishing aromaticity. The lone pairs are labeled A-D below.
Chapter 11 Solutions
Organic Chemistry
Ch. 11 - Problem 11.1 Neopheliosyne B is a novel acetylenic...Ch. 11 - Give the IUPAC name for each compound.Ch. 11 - Give the structures corresponding to each of the...Ch. 11 - Prob. 11.4PCh. 11 - Prob. 11.5PCh. 11 - Which bases can deprotonate acetylene? The pKa...Ch. 11 - Draw the organic products formed when each alkyne...Ch. 11 - Draw additional resonance structures for each...Ch. 11 - Problem 11.9 Draw the products formed when is...Ch. 11 - Explain the following result. Although alkenes...
Ch. 11 - Problem 11.11 Draw the keto tautomer of each...Ch. 11 - Prob. 11.12PCh. 11 - a Draw two different enol tautomers of...Ch. 11 - Prob. 11.14PCh. 11 - Problem 11.15 Draw the organic products formed in...Ch. 11 - Problem 11.16 What acetylide anion and alkyl...Ch. 11 - Problem. 11.17 Show how , and can be used to...Ch. 11 - Prob. 11.18PCh. 11 - Draw the products of each reaction. a. b.Ch. 11 - Prob. 11.20PCh. 11 - Problem 11.21 Use retrosynthetic analysis to show...Ch. 11 - Prob. 11.22PCh. 11 - Give the IUPAC name for each compound. a. b.Ch. 11 - Prob. 11.24PCh. 11 - 11.25 Answer the following questions about...Ch. 11 - 11.26 Give the IUPAC name for each alkyne.
a. ...Ch. 11 - Prob. 11.27PCh. 11 - Which of the following pairs of compounds...Ch. 11 - Prob. 11.29PCh. 11 - 11.30 How is each compound related to A? Choose...Ch. 11 - Prob. 11.31PCh. 11 - Prob. 11.32PCh. 11 - 11.33 Draw the products formed when is treated...Ch. 11 - What reagents are needed to convert (CH3CH2)3CCCH...Ch. 11 - Prob. 11.35PCh. 11 - 11.36 What alkynes give each of the following...Ch. 11 - 11.37 What alkyne gives each compound as the only...Ch. 11 - 11.38 Draw the organic products formed in each...Ch. 11 - 11.39 Draw the structure of compounds A-E in the...Ch. 11 - Prob. 11.40PCh. 11 - Prob. 11.41PCh. 11 - 11.42 What reactions are needed to convert alcohol...Ch. 11 - Prob. 11.43PCh. 11 - Prob. 11.44PCh. 11 - 11.45 Explain the following statement. Although ...Ch. 11 - 11.46 Tautomerization in base resembles...Ch. 11 - 11.47 Draw a stepwise mechanism for each...Ch. 11 - Prob. 11.48PCh. 11 - Prob. 11.49PCh. 11 - 11.50 What acetylide anion and alkyl halide are...Ch. 11 - 11.51 Synthesize each compound from acetylene. You...Ch. 11 - 11.52 Devise a synthesis of each compound using ...Ch. 11 - Prob. 11.53PCh. 11 - Prob. 11.54PCh. 11 - 11.55 Devise a synthesis of the ketone, , from ...Ch. 11 - 11.56 Devise a synthesis of each compound using ...Ch. 11 - Prob. 11.57PCh. 11 - Prob. 11.58PCh. 11 - 11.59 N-Chlorosuccinimide (NCS) serves as a source...Ch. 11 - 11.60 Draw a stepwise mechanism for the following...Ch. 11 - 11.61 Draw a stepwise mechanism for the following...Ch. 11 - Prob. 11.62PCh. 11 - 11.63 Write a stepwise mechanism for each of the...Ch. 11 - Prob. 11.64PCh. 11 - 11.65 Explain why an optically active solution of ...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Gregor Mendel never saw a gene, yet he concluded that some inherited factors were responsible for the patterns ...
Campbell Essential Biology (7th Edition)
Choose the best answer to each of the following. Explain your reasoning. If Earth were twice as far as it actua...
Cosmic Perspective Fundamentals
11. In the early 1800s, French naturalist Jean Baptiste Lamarck suggested that the best explanation for the rel...
Campbell Biology: Concepts & Connections (9th Edition)
An obese 55-year-old woman consults her physician about minor chest pains during exercise. Explain the physicia...
Biology: Life on Earth with Physiology (11th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A 0.10 M solution of acetic acid (CH3COOH, Ka = 1.8 x 10^-5) is titrated with a 0.0250 M solution of magnesium hydroxide (Mg(OH)2). If 10.0 mL of the acid solution is titrated with 10.0 mL of the base solution, what is the pH of the resulting solution?arrow_forwardGiven a complex reaction with rate equation v = k1[A] + k2[A]2, what is the overall reaction order?arrow_forwardPlease draw the structure in the box that is consistent with all the spectral data and alphabetically label all of the equivalent protons in the structure (Ha, Hb, Hc....) in order to assign all the proton NMR peaks. The integrations are computer generated and approximate the number of equivalent protons. Molecular formula: C13H1802 14 13 12 11 10 11 (ppm) Structure with assigned H peaks 2.08 3.13arrow_forward
- CHEMICAL KINETICS. One of the approximation methods for solving the rate equation is the steady-state approximation method. Explain what it consists of.arrow_forwardCHEMICAL KINETICS. One of the approximation methods for solving the rate equation is the limiting or determining step approximation method. Explain what it consists of.arrow_forwardCHEMICAL KINETICS. Indicate the approximation methods for solving the rate equation.arrow_forward
- TRANSMITTANCE เบบ Please identify the one structure below that is consistent with the 'H NMR and IR spectra shown and draw its complete structure in the box below with the protons alphabetically labeled as shown in the NMR spectrum and label the IR bands, including sp³C-H and sp2C-H stretch, indicated by the arrows. D 4000 OH LOH H₂C CH3 OH H₂C OCH3 CH3 OH 3000 2000 1500 HAVENUMBERI-11 1000 LOCH3 Draw your structure below and label its equivalent protons according to the peak labeling that is used in the NMR spectrum in order to assign the peaks. Integrals indicate number of equivalent protons. Splitting patterns are: s=singlet, d=doublet, m-multiplet 8 3Hb s m 1Hd s 3Hf m 2Hcd 2Had 1He 鄙视 m 7 7 6 5 4 3 22 500 T 1 0arrow_forwardRelative Transmittance 0.995 0.99 0.985 0.98 Please draw the structure that is consistent with all the spectral data below in the box and alphabetically label the equivalent protons in the structure (Ha, Hb, Hc ....) in order to assign all the proton NMR peaks. Label the absorption bands in the IR spectrum indicated by the arrows. INFRARED SPECTRUM 1 0.975 3000 2000 Wavenumber (cm-1) 1000 Structure with assigned H peaks 1 3 180 160 140 120 100 f1 (ppm) 80 60 40 20 0 C-13 NMR note that there are 4 peaks between 120-140ppm Integral values equal the number of equivalent protons 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 2.0 1.0 0.0 fl (ppm)arrow_forwardCalculate the pH of 0.0025 M phenol.arrow_forward
- In the following reaction, the OH- acts as which of these? NO2-(aq) + H2O(l) ⇌ OH-(aq) + HNO2(aq)arrow_forwardUsing spectra attached, can the unknown be predicted? Draw the predicition. Please explain and provide steps. Molecular focrmula:C16H13ClOarrow_forwardCalculate the percent ionization for 0.0025 M phenol. Use the assumption to find [H3O+] first. K = 1.0 x 10-10arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY