
Concept explainers
Neopheliosyne B is a novel acetylenic fatty acid isolated from a New Caledonian marine sponge. (a) Label the most acidic H atom. (b) Which carbon-carbon
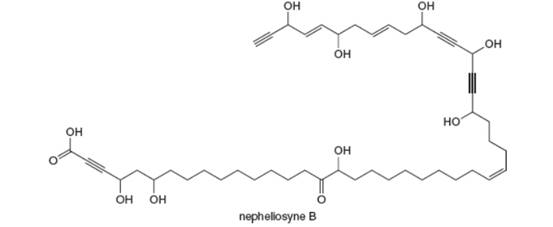
(a)
Interpretation: The most acidic H atom present in nepheliosyne B is to be labeled.
Concept introduction: The most acidic hydrogen
Answer to Problem 11.1P
The labeling of the most acidic H-atom present in nepheliosyne B is shown below.
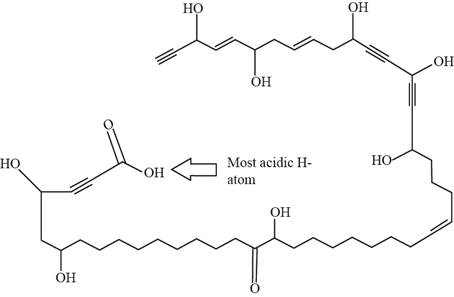
Explanation of Solution
The labeling of most acidic H-atom is shown as,
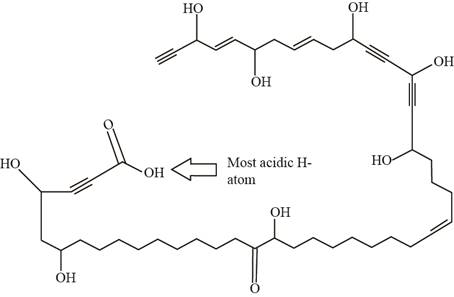
Figure 1
The stability of conjugate base of carboxylic acid is more than the stability of alcohol’s conjugate base. Thus, the acidity of hydrogen atom present in the carboxylic acid is more than that of alcohol because resonance stabilized conjugate base results in an increase in the acidity of a compound.
The labeling of most acidic H-atom present in nepheliosyne B is shown in Figure 1.
(b)
Interpretation: The shortest carbon-carbon
Concept introduction: The higher value of s-character corresponds to the higher electronegativity and lesser bond strength The highly electronegative carbon that is
Answer to Problem 11.1P
The shortest carbon-carbon
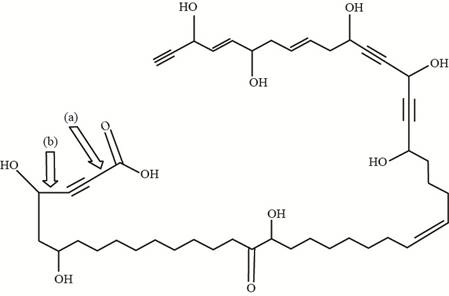
Explanation of Solution
The carbon-carbon
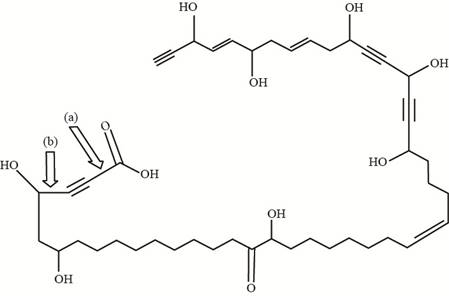
Figure 2
The bond (a) in the given compound is formed by
The shortest carbon-carbon
(c)
Interpretation: The number of degree of unsaturation contained by nepheliosyne B is to be stated.
Concept introduction: The degree of unsaturation states the total number of double bonds, triple bonds or rings present in a compound. It is calculated by the molecular formula of compound containing carbon and hydrogen atom.
Answer to Problem 11.1P
There are total
Explanation of Solution
The molecular formula of nepheliosyne B is given by,
Where
•
Substitute the value of
As the molecular formula of compound contains
The degree of unstauration is calculated by the formula,
Hence, the total number of degree of unstauration present in nepheliosyne B is
The total number of degree of unstauration present in nepheliosyne B is
(d)
Interpretation: The number of bonds formed by
Concept introduction: The procedure of intermixing of the atomic orbitals in an atom is known hybridization. This intermixing of the atomic orbitals is used to form a set of new atomic orbital with different geometry.
Answer to Problem 11.1P
The number of bonds formed by
Explanation of Solution
The number of bonds formed by
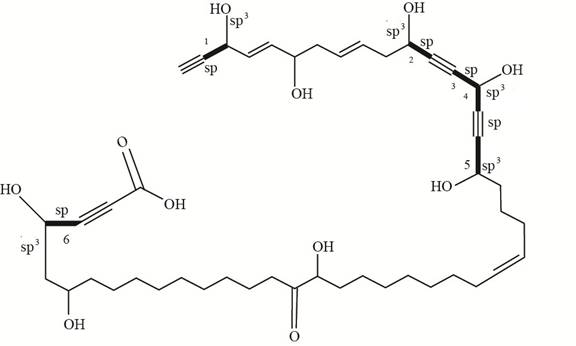
Figure 3
Thus, there are six bonds formed by
The total number of bonds formed by
(e)
Interpretation: Each of the triple bond present in nepheliosyne B is to be labeled as internal or terminal.
Concept introduction: The triple bond or alkyne which contains carbon substituents over acetylenic carbon is known as internal triple bond or alkyne. In case of terminal triple bond or alkyne, there is at least one acetylenic carbon that contains H-atom linked to it.
Answer to Problem 11.1P
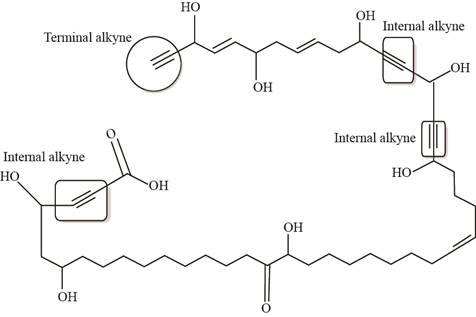
The labeling of the triple bonds present in nepheliosyne B as internal or terminal is shown as,
Explanation of Solution
The labeling of the triple bonds present in nepheliosyne B as internal or terminal is shown as,

Figure 4
In Figure 4, there are three internal triple bonds and one terminal triple bond present.
The labeling of the triple bonds present in nepheliosyne B as internal or terminal is shown in Figure 4.
Want to see more full solutions like this?
Chapter 11 Solutions
Organic Chemistry
Additional Science Textbook Solutions
General, Organic, and Biological Chemistry - 4th edition
Campbell Biology: Concepts & Connections (9th Edition)
Campbell Essential Biology (7th Edition)
Brock Biology of Microorganisms (15th Edition)
Human Physiology: An Integrated Approach (8th Edition)
- Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. H H CH3OH, H+ H Select to Add Arrows H° 0:0 'H + Q HH ■ Select to Add Arrows CH3OH, H* H. H CH3OH, H+ HH ■ Select to Add Arrows i Please select a drawing or reagent from the question areaarrow_forwardWhat are examples of analytical methods that can be used to analyse salt in tomato sauce?arrow_forwardA common alkene starting material is shown below. Predict the major product for each reaction. Use a dash or wedge bond to indicate the relative stereochemistry of substituents on asymmetric centers, where applicable. Ignore any inorganic byproducts H Šali OH H OH Select to Edit Select to Draw 1. BH3-THF 1. Hg(OAc)2, H2O =U= 2. H2O2, NaOH 2. NaBH4, NaOH + Please select a drawing or reagent from the question areaarrow_forward
- What is the MOHR titration & AOAC method? What is it and how does it work? How can it be used to quantify salt in a sample?arrow_forwardPredict the major products of this reaction. Cl₂ hv ? Draw only the major product or products in the drawing area below. If there's more than one major product, you can draw them in any arrangement you like. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry. If there will be no products because there will be no significant reaction, just check the box under the drawing area and leave it blank. Note for advanced students: you can ignore any products of repeated addition. Explanation Check Click and drag to start drawing a structure. 80 10 m 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility DII A F1 F2 F3 F4 F5 F6 F7 F8 EO F11arrow_forwardGiven a system with an anodic overpotential, the variation of η as a function of current density- at low fields is linear.- at higher fields, it follows Tafel's law.Calculate the range of current densities for which the overpotential has the same value when calculated for both cases (the maximum relative difference will be 5%, compared to the behavior for higher fields).arrow_forward
- Using reaction free energy to predict equilibrium composition Consider the following equilibrium: N2 (g) + 3H2 (g) = 2NH3 (g) AGº = -34. KJ Now suppose a reaction vessel is filled with 8.06 atm of nitrogen (N2) and 2.58 atm of ammonia (NH3) at 106. °C. Answer the following questions about this system: rise Under these conditions, will the pressure of N2 tend to rise or fall? ☐ x10 fall Is it possible to reverse this tendency by adding H₂? In other words, if you said the pressure of N2 will tend to rise, can that be changed to a tendency to fall by adding H2? Similarly, if you said the pressure of N will tend to fall, can that be changed to a tendency to rise by adding H₂? If you said the tendency can be reversed in the second question, calculate the minimum pressure of H₂ needed to reverse it. Round your answer to 2 significant digits. yes no ☐ atm Х ด ? olo 18 Ararrow_forwardFour liters of an aqueous solution containing 6.98 mg of acetic acid were prepared. At 25°C, the measured conductivity was 5.89x10-3 mS cm-1. Calculate the degree of dissociation of the acid and its ionization constant.Molecular weights: O (15.999), C (12.011), H (1.008).Limiting molar ionic conductivities (λ+0 and λ-0) of Ac-(aq) and H+(aq): 40.9 and 349.8 S cm-2 mol-1.arrow_forwardDetermine the change in Gibbs energy, entropy, and enthalpy at 25°C for the battery from which the data in the table were obtained.T (°C) 15 20 25 30 35Eo (mV) 227.13 224.38 221.87 219.37 216.59Data: n = 1, F = 96485 C mol–1arrow_forward
- Indicate the correct options.1. The units of the transport number are Siemens per mole.2. The Siemens and the ohm are not equivalent.3. The Van't Hoff factor is dimensionless.4. Molar conductivity does not depend on the electrolyte concentration.arrow_forwardIdeally nonpolarizable electrodes can1. participate as reducers in reactions.2. be formed only with hydrogen.3. participate as oxidizers in reactions.4. form open and closed electrochemical systems.arrow_forwardIndicate the options for an electrified interface:1. Temperature has no influence on it.2. Not all theories that describe it include a well-defined electrical double layer.3. Under favorable conditions, its differential capacitance can be determined with the help of experimental measurements.4. A component with high electronic conductivity is involved in its formation.arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

