
Organic Chemistry
5th Edition
ISBN: 9780078021558
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 11, Problem 11.11P
Draw the keto tautomer of each enol.
a. 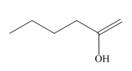 b.
b. 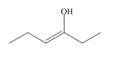 c.
c. 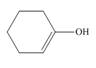
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Using the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$
Indicate characteristics of oxodec acid.
What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.
Chapter 11 Solutions
Organic Chemistry
Ch. 11 - Problem 11.1 Neopheliosyne B is a novel acetylenic...Ch. 11 - Give the IUPAC name for each compound.Ch. 11 - Give the structures corresponding to each of the...Ch. 11 - Prob. 11.4PCh. 11 - Prob. 11.5PCh. 11 - Which bases can deprotonate acetylene? The pKa...Ch. 11 - Draw the organic products formed when each alkyne...Ch. 11 - Draw additional resonance structures for each...Ch. 11 - Problem 11.9 Draw the products formed when is...Ch. 11 - Explain the following result. Although alkenes...
Ch. 11 - Problem 11.11 Draw the keto tautomer of each...Ch. 11 - Prob. 11.12PCh. 11 - a Draw two different enol tautomers of...Ch. 11 - Prob. 11.14PCh. 11 - Problem 11.15 Draw the organic products formed in...Ch. 11 - Problem 11.16 What acetylide anion and alkyl...Ch. 11 - Problem. 11.17 Show how , and can be used to...Ch. 11 - Prob. 11.18PCh. 11 - Draw the products of each reaction. a. b.Ch. 11 - Prob. 11.20PCh. 11 - Problem 11.21 Use retrosynthetic analysis to show...Ch. 11 - Prob. 11.22PCh. 11 - Give the IUPAC name for each compound. a. b.Ch. 11 - Prob. 11.24PCh. 11 - 11.25 Answer the following questions about...Ch. 11 - 11.26 Give the IUPAC name for each alkyne.
a. ...Ch. 11 - Prob. 11.27PCh. 11 - Which of the following pairs of compounds...Ch. 11 - Prob. 11.29PCh. 11 - 11.30 How is each compound related to A? Choose...Ch. 11 - Prob. 11.31PCh. 11 - Prob. 11.32PCh. 11 - 11.33 Draw the products formed when is treated...Ch. 11 - What reagents are needed to convert (CH3CH2)3CCCH...Ch. 11 - Prob. 11.35PCh. 11 - 11.36 What alkynes give each of the following...Ch. 11 - 11.37 What alkyne gives each compound as the only...Ch. 11 - 11.38 Draw the organic products formed in each...Ch. 11 - 11.39 Draw the structure of compounds A-E in the...Ch. 11 - Prob. 11.40PCh. 11 - Prob. 11.41PCh. 11 - 11.42 What reactions are needed to convert alcohol...Ch. 11 - Prob. 11.43PCh. 11 - Prob. 11.44PCh. 11 - 11.45 Explain the following statement. Although ...Ch. 11 - 11.46 Tautomerization in base resembles...Ch. 11 - 11.47 Draw a stepwise mechanism for each...Ch. 11 - Prob. 11.48PCh. 11 - Prob. 11.49PCh. 11 - 11.50 What acetylide anion and alkyl halide are...Ch. 11 - 11.51 Synthesize each compound from acetylene. You...Ch. 11 - 11.52 Devise a synthesis of each compound using ...Ch. 11 - Prob. 11.53PCh. 11 - Prob. 11.54PCh. 11 - 11.55 Devise a synthesis of the ketone, , from ...Ch. 11 - 11.56 Devise a synthesis of each compound using ...Ch. 11 - Prob. 11.57PCh. 11 - Prob. 11.58PCh. 11 - 11.59 N-Chlorosuccinimide (NCS) serves as a source...Ch. 11 - 11.60 Draw a stepwise mechanism for the following...Ch. 11 - 11.61 Draw a stepwise mechanism for the following...Ch. 11 - Prob. 11.62PCh. 11 - 11.63 Write a stepwise mechanism for each of the...Ch. 11 - Prob. 11.64PCh. 11 - 11.65 Explain why an optically active solution of ...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Choose the best answer to each of the following. Explain your reasoning. If Earth were twice as far as it actua...
Cosmic Perspective Fundamentals
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
6. How can you use the features found in each chapter?
Human Anatomy & Physiology (2nd Edition)
Separate the list P,F,V,,T,a,m,L,t, and V into intensive properties, extensive properties, and nonproperties.
Fundamentals Of Thermodynamics
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forward
- please help me with my homeworkarrow_forwardhelparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forward
- QUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forwarder your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY