
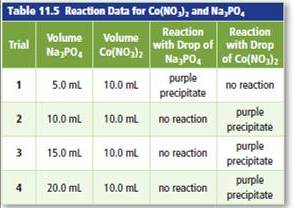
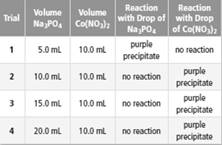
Apply Students conducted a lab to investigate limiting and excess reactants. The students added different volumes of sodium phosphate solution
a. Write a balanced chemical equation for the reaction.
b. Based on the results, identify the limiting reactant and the excess reactant for each trial.
(a)
Interpretation:
The balance chemical equation between sodium phosphate and cobalt (III) nitrate to form purple precipitate needs to be determined.
Concept introduction:
A chemical reaction involves the conversion of certain molecules (reactant) to new substance (product) by bond breaking and making. One of the reactant, which is present in limited amount and determines the amount of product, called as limiting reactant. Whereas another reactant is called as excess reactant.
Answer to Problem 108A
Explanation of Solution
The reaction between sodium phosphate and cobalt (III) nitrate forms purple precipitate. The balance chemical reaction can be written as:
Here the purple precipitate is formed due to cobalt (III) phosphate and sodium nitrate will remain in solution.
(b)
Interpretation:
The limiting and excess reactant for the reaction between sodium phosphate and cobalt (III) nitrate to form purple precipitate needs to be determined.
Concept introduction:
A chemical reaction involves the conversion of certain molecules (reactant) to new substance (product) by bond breaking and making. One of the reactant, which is present in limited amount and determines the amount of product, called as limiting reactant. Whereas another reactant is called as excess reactant.
Answer to Problem 108A
Trial 1 =
In the trials 2 to 4 =
Explanation of Solution
The reaction between sodium phosphate and cobalt (III) nitrate forms purple precipitate. The balance chemical reaction can be written as:
According to trial 1,
In the trials 2 to 4
Chapter 11 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Chemistry: Structure and Properties (2nd Edition)
Chemistry: The Central Science (14th Edition)
CHEMISTRY-TEXT
Chemistry: Structure and Properties
General Chemistry: Principles and Modern Applications (11th Edition)
General, Organic, and Biological Chemistry (3rd Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





