
Concept explainers
Arrange the compounds of each of the following series in order of increasing acidity:
Interpretation:
The compounds of each of the given series are to be arranged in order of increasing acidity.
Concept introduction:
Acidity depends on the
Resonance structures are the structures in which two or more possible electron structures are drawn. In the resonance structure, the position of the atoms is the same but position of the electrons is different.
Resonance causes delocalization of electron pairs, which increases the stability of the base.
Answer to Problem 1P
Solution:
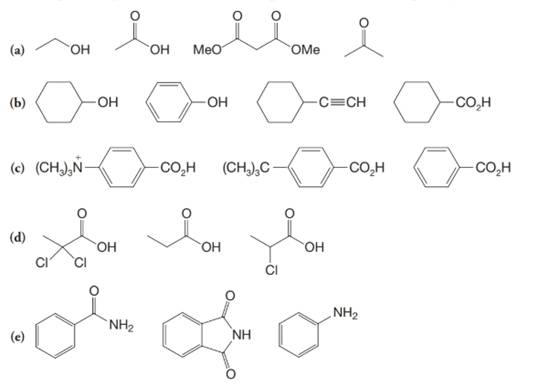
(a)
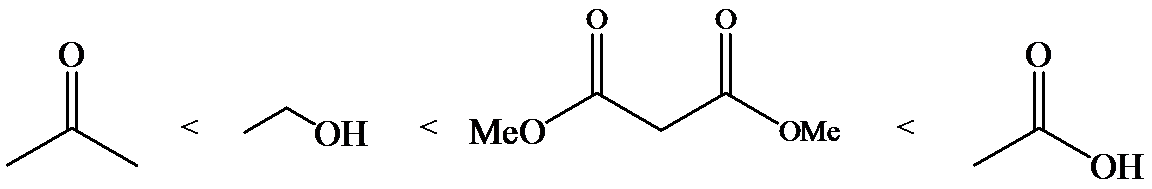
(b)
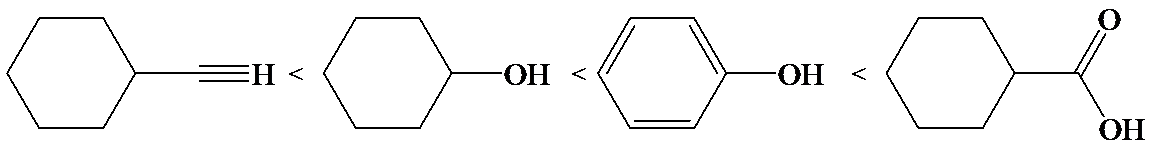
(c)

(d)
(e)
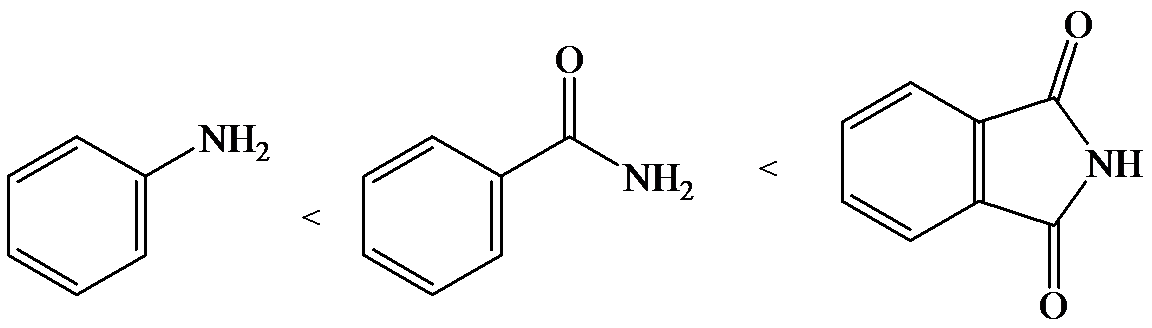
Explanation of Solution
a)
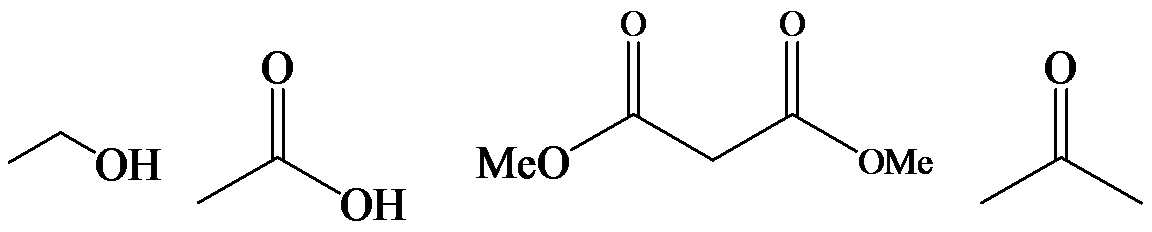
The α-hydrogen atoms of carbonyl groups are acidic. The acidity arises from the electron withdrawing effect of the carbonyl and resonance stabilization of its conjugate base. The electron donating effect of 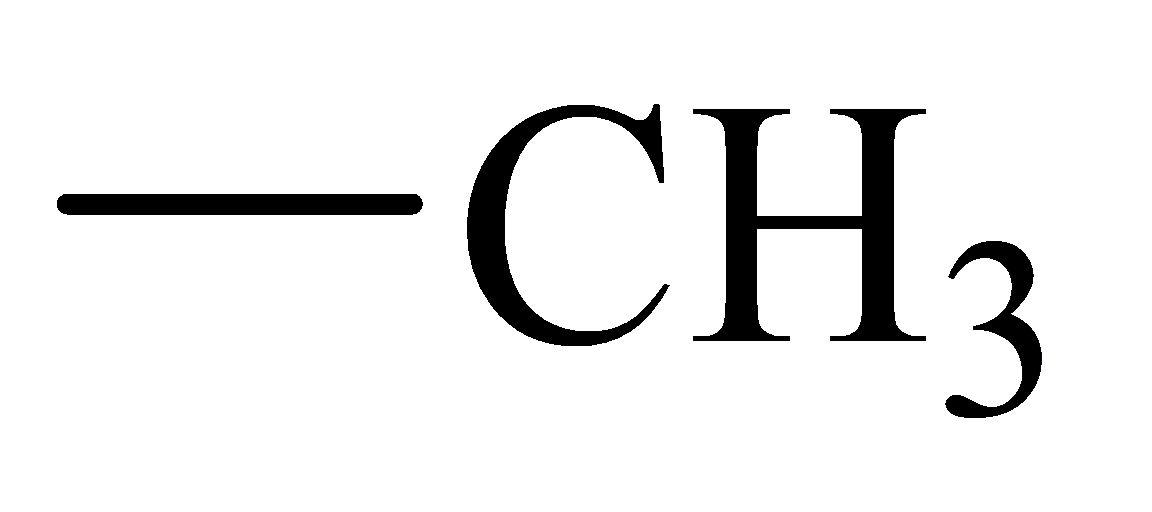 groups tends to destabilize anions. In diketone, there is an active methylene, adjacent to two carbonyl groups. This indicates more resonance stabilization. The charge of anion can be delocalized to both oxygen atoms. The hydroxyl proton in carboxylic acid is an α-proton. On comparing the acidity of carboxylic acids and alcohols, alcohol is less acidic than carboxylic acid.
groups tends to destabilize anions. In diketone, there is an active methylene, adjacent to two carbonyl groups. This indicates more resonance stabilization. The charge of anion can be delocalized to both oxygen atoms. The hydroxyl proton in carboxylic acid is an α-proton. On comparing the acidity of carboxylic acids and alcohols, alcohol is less acidic than carboxylic acid.
So, the increasing order of the acidity is as follows:
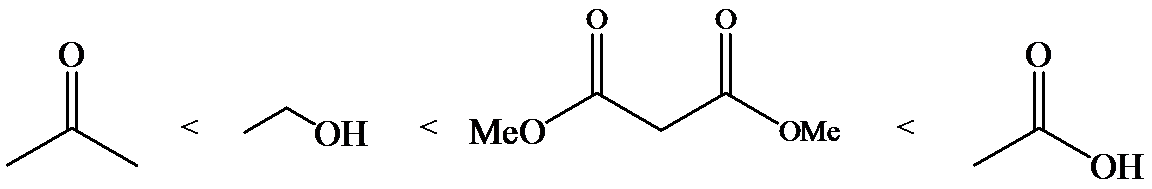
b)
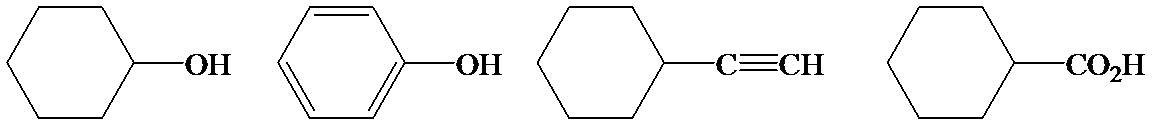
Phenol is more acidic than cyclohexanol because the resonance stabilization in both is different.
In the case of cyclohexane carboxylic acid, negative charge is shared between two different oxygen atoms making it more stabilized than phenoxide. Hence, the removal of proton from cyclohexane carboxylic acid is easier than phenol, making it more acidic than phenol.
So, the increasing order of the acidity is as follows:
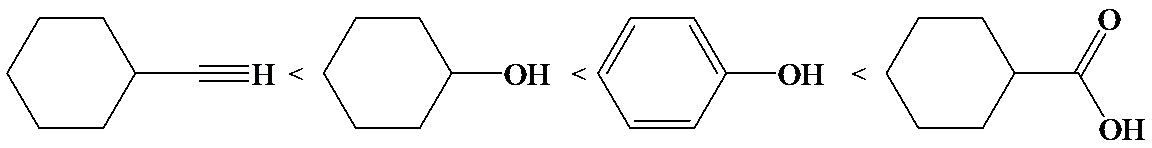
c)
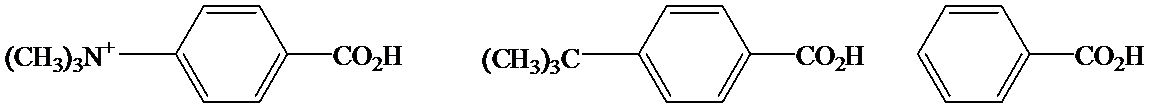
In the case of carboxylic acids, electron substituents increase acidity by inductive electron donation. The electron-donating tert-butyl group destabilizes the conjugate base of benzoic acid, making it less acidic.
So, the increasing order of the acidity is as follows:

d)
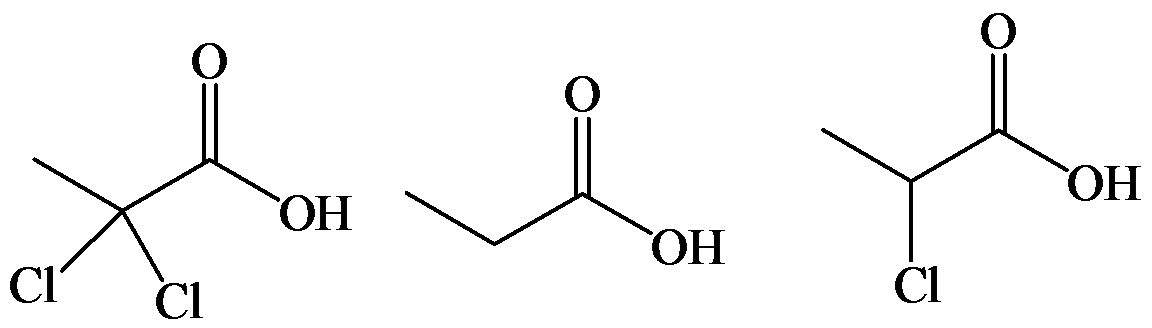
The electron-withdrawing chloro groups increase the acidity of carboxylic acid by increasing the stability of the carboxylate ion. Hence, the carboxylic acid with more chloro groups is more acidic.
So, the increasing order of the acidity is as follows:
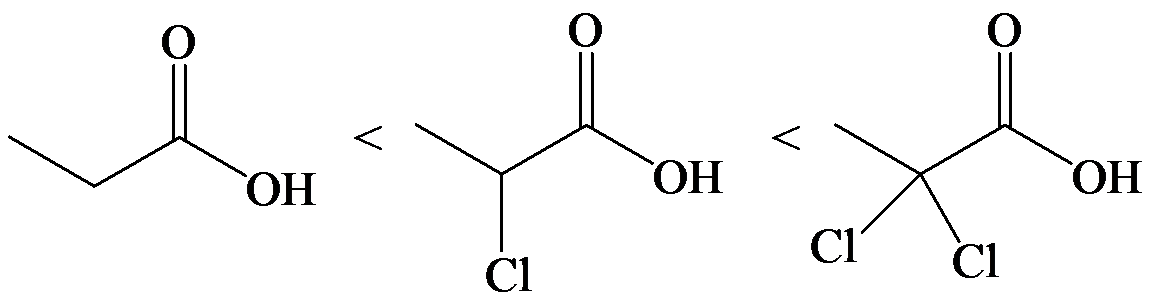
e)
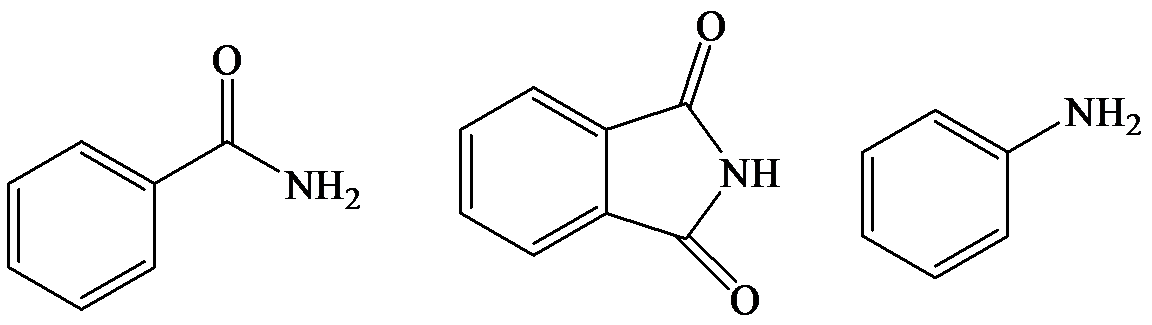
The lone pair electron in aniline is localized on the nitrogen atom, whereas onbenzamide, it is delocalized between oxygen and nitrogen via resonance. Therefore, benzamide is more acidic than aniline.
So, the increasing order of the acidity is as follows:
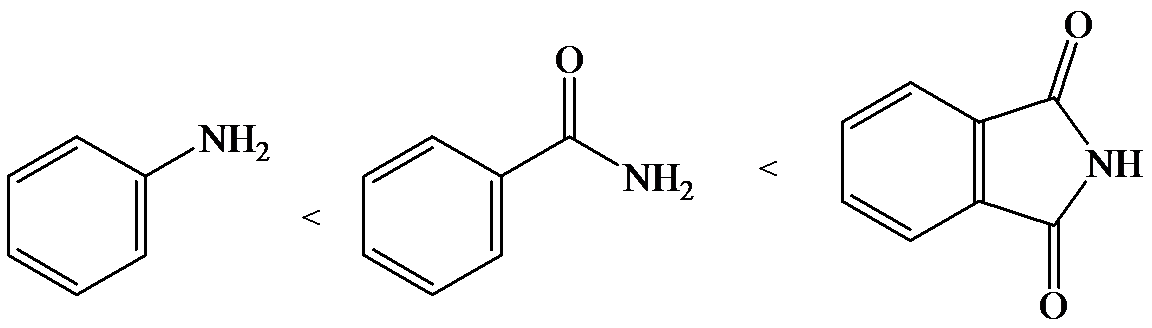
Want to see more full solutions like this?
Chapter SRP Solutions
Organic Chemistry
Additional Science Textbook Solutions
Chemistry: Structure and Properties (2nd Edition)
Human Anatomy & Physiology (2nd Edition)
Microbiology: An Introduction
Human Biology: Concepts and Current Issues (8th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Cosmic Perspective Fundamentals
- Select the stronger base from each pair of compounds. (a) H₂CNH₂ or EtzN (b) CI or NH2 NH2 (c) .Q or EtzN (d) or (e) N or (f) H or Harrow_forward4. Provide a clear arrow-pushing mechanism for each of the following reactions. Do not skip proton transfers, do not combine steps, and make sure your arrows are clear enough to be interpreted without ambiguity. a. 2. 1. LDA 3. H3O+ HOarrow_forwardb. H3C CH3 H3O+ ✓ H OHarrow_forward
- 2. Provide reagents/conditions to accomplish the following syntheses. More than one step is required in some cases. a. CH3arrow_forwardIdentify and provide an explanation that distinguishes a qualitative and quantitative chemical analysis. Provide examples.arrow_forwardIdentify and provide an explanation of the operational principles behind a Atomic Absorption Spectrometer (AAS). List the steps involved.arrow_forward
- Instructions: Complete the questions in the space provided. Show all your work 1. You are trying to determine the rate law expression for a reaction that you are completing at 25°C. You measure the initial reaction rate and the starting concentrations of the reactions for 4 trials. BrO³¯ (aq) + 5Br¯ (aq) + 6H* (aq) → 3Br₂ (l) + 3H2O (l) Initial rate Trial [BrO3] [H*] [Br] (mol/L) (mol/L) | (mol/L) (mol/L.s) 1 0.10 0.10 0.10 8.0 2 0.20 0.10 0.10 16 3 0.10 0.20 0.10 16 4 0.10 0.10 0.20 32 a. Based on the above data what is the rate law expression? b. Solve for the value of k (make sure to include proper units) 2. The proposed reaction mechanism is as follows: i. ii. BrО¸¯ (aq) + H+ (aq) → HBrO3 (aq) HBrO³ (aq) + H* (aq) → H₂BrO3* (aq) iii. H₂BrO³* (aq) + Br¯ (aq) → Br₂O₂ (aq) + H2O (l) [Fast] [Medium] [Slow] iv. Br₂O₂ (aq) + 4H*(aq) + 4Br(aq) → 3Br₂ (l) + H2O (l) [Fast] Evaluate the validity of this proposed reaction. Justify your answer.arrow_forwardе. Д CH3 D*, D20arrow_forwardC. NaOMe, Br Brarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

