
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter SRP, Problem 19P
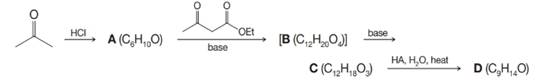
Give structures for compounds A–D. Compound D gives a strong IR absorption band near
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
When talking about the acidity of carboxylic acids, is it the same thing to say higher or stronger acidity?
Using the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$
Indicate characteristics of oxodec acid.
Chapter SRP Solutions
Organic Chemistry
Ch. SRP - 1. Arrange the compounds of each of the following...Ch. SRP - 2. Arrange the compounds of each of the following...Ch. SRP - Predict the final product from each of the...Ch. SRP - Prob. 4PCh. SRP - Write detailed mechanisms for each of the...Ch. SRP - Prob. 6PCh. SRP - Prob. 7PCh. SRP - Prob. 8PCh. SRP - Prob. 9PCh. SRP - Give stereochemical structures for compounds AD:
Ch. SRP - Prob. 11PCh. SRP - The remaining steps in the industrial synthesis of...Ch. SRP - Prob. 13PCh. SRP - Prob. 14PCh. SRP - Prob. 15PCh. SRP - Prob. 16PCh. SRP - 17. Show how you would modify the synthesis given...Ch. SRP - Prob. 18PCh. SRP - Give structures for compounds AD. Compound D gives...Ch. SRP - The tranquilizing drug meprobamate (Equanil or...Ch. SRP - Prob. 21PCh. SRP - 22. Outlined here is the synthesis of a central...Ch. SRP - 23. What are compounds A and B? Compound B has a...Ch. SRP - Prob. 24PCh. SRP - 25. The Dow process for synthesizing phenol, which...Ch. SRP - Prob. 26PCh. SRP - Prob. 27PCh. SRP - 28. Compound Y shows prominent IR absorption...Ch. SRP - Prob. 29PCh. SRP - Consider this reaction involving peracetic acid:...Ch. SRP - 31. A compound (N) with the molecular formula...Ch. SRP - 32. Compound X is insoluble in aqueous sodium...Ch. SRP - Write the structures of the three products...Ch. SRP - Compound C (C9H11NO) gives a positive Tollens test...Ch. SRP - A compound X (C10H14O) dissolves in aqueous sodium...Ch. SRP - Compound Z (C5H10O) decolorizes bromine. The IR...Ch. SRP - 37. Compound W was isolated from a marine annelid...Ch. SRP - 38. Phenols generally are not changed on treatment...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Assume that genes, A and B are on the same chromosome and are 50 map units apart. An animal heterozygous at bot...
Campbell Biology (11th Edition)
27. Consider the reaction.
Express the rate of the reaction in terms of the change in concentration of each of...
Chemistry: Structure and Properties (2nd Edition)
Identify each of the following reproductive barriers as prezygotic or postzygotic. a. One lilac species lives o...
Campbell Essential Biology with Physiology (5th Edition)
Name the components (including muscles) of the thoracic cage. List the contents of the thorax.
Human Physiology: An Integrated Approach (8th Edition)
What type of cut would separate the brain into anterior and posterior parts?
Anatomy & Physiology (6th Edition)
1.2 Ask two of your friends (not in class) to define the terms in problem1.1.
Do their answers agee with the d...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.arrow_forwardWhat is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forward
- The temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY