
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter FRP, Problem 11P
Predict the products from each of the following reactions.
(a)
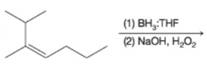
(b)
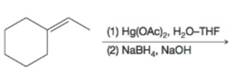
(c)
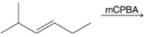
(d)
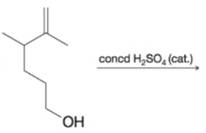
(e)
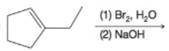
(f)
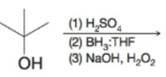
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
pls help
Indicate the product of the reaction
OH OH
CH3-CC- Ph + H2SO4 a 20°C
|
CH3 Ph
pls help
Chapter FRP Solutions
Organic Chemistry
Ch. FRP - Prob. 1PCh. FRP - 2. Which member of these pairs is the more polar?...Ch. FRP - Prob. 3PCh. FRP - Describe how solubility could be used to...Ch. FRP - 5. Though they each contain only one type of...Ch. FRP - Predict the products from each of the following...Ch. FRP - Prob. 7PCh. FRP - Prob. 8PCh. FRP - Prob. 9PCh. FRP - Prob. 10P
Ch. FRP - Predict the products from each of the following...Ch. FRP - Prob. 12PCh. FRP - 13. Starting with propyne and using any other...Ch. FRP - Bromination of 2-methylbutane yields predominantly...Ch. FRP - Prob. 15PCh. FRP - Account for the following observations with...Ch. FRP - Prob. 17PCh. FRP - Prob. 18PCh. FRP - Heating 1, 1,1-triphenylmethanol with ethanol...Ch. FRP - (a) Which of the following halides would you...Ch. FRP - An alkane (A) with the formula C6H14 reacts with...Ch. FRP - Prob. 22PCh. FRP - Prob. 23PCh. FRP - Dehydrohalogenation of meso-1, 2-dibromo-1,...Ch. FRP - Prob. 25PCh. FRP - Prob. 26PCh. FRP - 27. (R)-3-Methyl-1-pentene is treated separately...Ch. FRP - Prob. 28PCh. FRP - Prob. 29PCh. FRP - Prob. 30PCh. FRP - Prob. 31PCh. FRP - Synthesize the following compound by a method that...Ch. FRP - Provide three methods that employ Grignard...Ch. FRP - 34. Compound Yexhibits one NMR signal at (a...Ch. FRP - Prob. 35PCh. FRP - 36. Compound X shows a strong IR absorption band...Ch. FRP - Prob. 37PCh. FRP - 38. In addition to more highly fluorinated...Ch. FRP - Fluorination of (R)-2-flurobutane yields a mixture...Ch. FRP - Prob. 40PCh. FRP - Prob. 41P
Additional Science Textbook Solutions
Find more solutions based on key concepts
Point Reyes, located in the bottom-left corner of the map, is a headland undergoing severe wave erosion. What t...
Applications and Investigations in Earth Science (9th Edition)
If a compound has a molecular ion with an odd-numbered mass, then the compound contains an odd number of nitrog...
Organic Chemistry (8th Edition)
3. Which of the following is a major functional characteristic of all organisms? (a) movement, (b) growth (c) m...
Human Anatomy & Physiology (Marieb, Human Anatomy & Physiology) Standalone Book
A source of electromagnetic radiation produces infrared light. Which of the following could be the wavelength ...
Chemistry: The Central Science (14th Edition)
Define histology.
Fundamentals of Anatomy & Physiology (11th Edition)
How do food chains and food webs differ? Which is the more accurate representation of feeding relationships in ...
Biology: Life on Earth (11th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- pls helparrow_forwardpls helparrow_forward35) Complete the following equation by drawing the line the structure of the products that are formed. Please note that in some cases more than one product is possible. You must draw all possible products to recive full marks! a. ethanol + 2-propanol + H2SO4 → b. OH conc. H2SO4 CH2 H3C CH + K2Cr2O7 C. d. H3C A pressure CH3 + H2 CH Pt catalystarrow_forward
- 21) The rate of reaction depends upon: a. the concentration and nature of reactants b. the temperature of the reaction C. whether or not a catalyst was used d. all of the above 22) A Maxwell-Boltzmann curve shows the distribution of molecular energies in a reaction system. When the temperature in this system is increased, the peak is a. higher and further to the right. b. higher and further to the left. c. lower and further to the right. d. lower and further to the left. 23) Which of the following correctly describes the reaction represented by the reaction below? CaCO3 (s) + energy → CaO (s) + CO2 (g) a. It is exothermic and the potential energy is greater in the reactants than the products. b. c. It is exothermic and the potential energy is greater in the products than the reactants. It is endothermic and the potential energy is greater in the products than the reactants. d. It is endothermic and the potential energy is equal for the products and reactants.arrow_forwardpls helparrow_forward27) Draw the energy level diagram and write the full and shorthand electron configuration for a neutral sulfur atom.arrow_forward
- Indicate whether these compounds are isomers, enantiomers, or tautomers. OCH OCH محمد ممدarrow_forward30) Substance A to E below are listed with several of their properties. The identities of the substances are identified in random order below: Iron, ethane, ethanol, sodium nitrate, graphite First classify each substance as either a polar covalent compound, non-polar covalent compound, ionic compound, metallic solid, or network solid. Write your predictions in the sixth coloumn of the chart, under "type of substance." Then, identify the identity of the substance in the last coloumn. Substance Melting Point Boiling Point Solubility in H₂O Electrical Conductivity Type of Substance Identity of Substance (°C) (°C) as: Solid, Liquids, Solution A -182 -88 Insoluble No/No/- B 1538 2862 Insoluble Yes/Yes/- C 308 380 Soluble Yes/Yes/Yes Ꭰ 3456 Insoluble No/-/- E -114 78 Soluble No/No/Noarrow_forwardpls helparrow_forward
- 28) Explain the process of galvanization. In your description, make sure to explain what metal is usually used for galvanization and why this metal used.arrow_forward29) Complete the following table Molecule H₂O NH3 Lewis Dot Diagram VSEPR Diagram Name of VSEPR Shapearrow_forward12) What is the best name to describe the shape of water molecule? a. Angular b. C. Tetrahedral Octahedral d. Trigonal pyramidal 13) Network solids are distinguished from metallic crystals in that: a. Network solids have charged ions, while metallic crystals do not. b. Network solids are composed of molecules, while metallic crystals only have one type of atom. C. Network solids are composed of non-metals. d. Network solids have much lower boiling points.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY