
Concept explainers
What reagents would you use to achieve each of the following transformations:

Interpretation:
Reagents that can be used to achieve the given transformation has to be identified.
Concept Introduction:
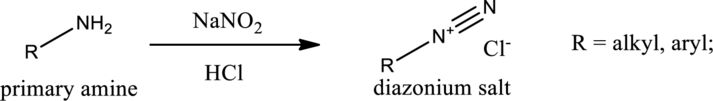
Amines react with nitrous acid to form diazonium salt. Nitrous acid is prepared in the reaction flask itself by the use of sodium nitrite and hydrochloric acid. Nitrous acid is protonated to produce highly reactive intermediate known as nitrosonium ion. Nitrosonium ion when reacted with amine results in the formation of nitrosoamine.
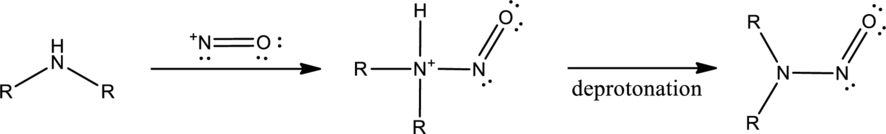
If a primary amine is considered, then tautomerization takes place resulting in formation of diazonium ion. This can be represented as,
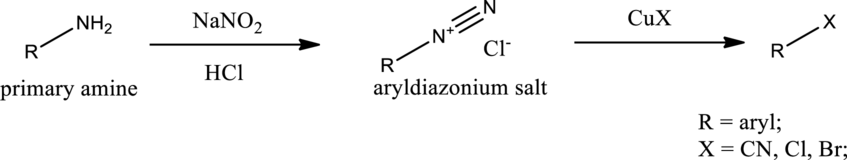
Sandmeyer reaction:
Aryldiazonium salt reacts with copper salts as reagent to obtain the required aryl halides, aryl cyanides etc. General scheme can be given as shown below,
Explanation of Solution
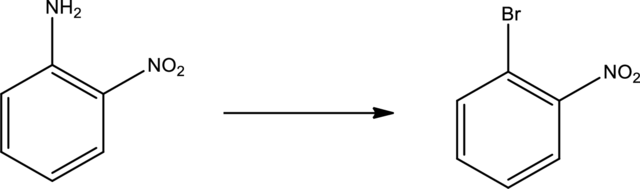
Given transformation is,
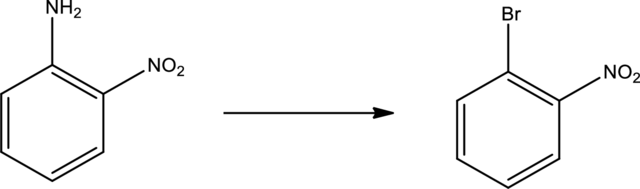
Product is an aryl halide while the starting material is an aryl amine. The route that can be used to accomplish the above reaction is the Sandmeyer reaction. In this the first step is the formation of aryl diazonium salt by the treatment of primary amine with sodium nitrite and hydrochloric acid. Second step is the treatment of aryldiazonium salt with copper bromide to form the aryl bromide.
The scheme can be given as shown below,
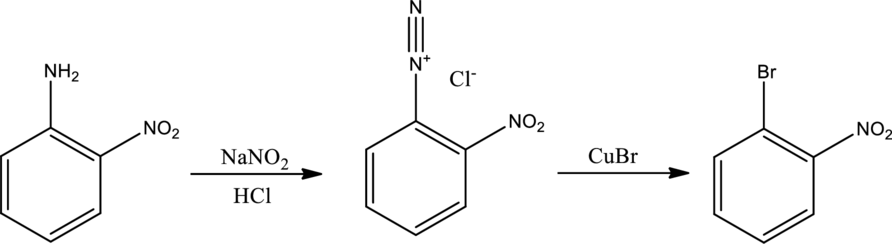
The reagents that are used are sodium nitrite with hydrochloric acid and copper(I) bromide.
Want to see more full solutions like this?
Chapter 9 Solutions
Organic Chemistry As a Second Language: Second Semester Topics
Additional Science Textbook Solutions
Introductory Chemistry (6th Edition)
Organic Chemistry (8th Edition)
Human Physiology: An Integrated Approach (8th Edition)
Campbell Biology in Focus (2nd Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Campbell Biology: Concepts & Connections (9th Edition)
- Nucleophilic Aromatic Substitution: What is the product of the reaction? *see imagearrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardThe answer here says that F and K have a singlet and a doublet. The singlet and doublet are referring to the H's 1 carbon away from the carbon attached to the OH. Why don't the H's two carbons away, the ones on the cyclohexane ring, cause more peaks on the signal?arrow_forward
- Draw the Birch Reduction for this aromatic compound and include electron withdrawing groups and electron donating groups. *See attachedarrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardBlocking Group are use to put 2 large sterically repulsive group ortho. Show the correct sequence toconnect the reagent to product with the highest yield possible. * see imagearrow_forward
- Elimination-Addition: What molecule was determined to be an intermediate based on a “trapping experiment”? *please solve and see imagearrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardPredict the final product. If 2 products are made, list which should be “major” and “minor”. **see attachedarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

