
Organic Chemistry As a Second Language: Second Semester Topics
4th Edition
ISBN: 9781119110651
Author: David R. Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9.2, Problem 9.7P
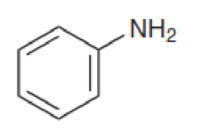
Identify whether each of the following compounds could be made with a Gabriel synthesis:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Blocking Group are use to put 2 large sterically repulsive group ortho. Show the correct sequence toconnect the reagent to product with the highest yield possible. * see image **NOTE: The compound on the left is the starting point, and the compound on the right is the final product. Please show the steps in between to get from start to final, please. These are not two different compounds that need to be worked.
I dont understand this.
Can you please explain this prooblem to me, show me how the conjugation is added, did I add them in the correct places and if so please show me. Thanks!
Chapter 9 Solutions
Organic Chemistry As a Second Language: Second Semester Topics
Ch. 9.2 - Identify how you would use a Gabriel synthesis to...Ch. 9.2 - Identify how you would use a Gabriel synthesis to...Ch. 9.2 - Identify how you would use a Gabriel synthesis to...Ch. 9.2 - Identify how you would use a Gabriel synthesis to...Ch. 9.2 - Identify whether each of the following compounds...Ch. 9.2 - Identify whether each of the following compounds...Ch. 9.2 - Identify whether each of the following compounds...Ch. 9.2 - Identify whether each of the following compounds...Ch. 9.3 - Suggest an efficient synthesis for each of the...Ch. 9.3 - Suggest an efficient synthesis for each of the...
Ch. 9.3 - Suggest an efficient synthesis for each of the...Ch. 9.3 - Suggest an efficient synthesis for each of the...Ch. 9.3 - Suggest an efficient synthesis for each of the...Ch. 9.3 - Prob. 9.18PCh. 9.3 - Prob. 9.19PCh. 9.3 - Prob. 9.20PCh. 9.3 - Prob. 9.21PCh. 9.3 - Prob. 9.22PCh. 9.4 - Prob. 9.24PCh. 9.4 - Prob. 9.25PCh. 9.5 - Predict the major product for each of the...Ch. 9.5 - Predict the major product for each of the...Ch. 9.5 - Predict the major product for each of the...Ch. 9.5 - Predict the major product for each of the...Ch. 9.6 - What reagents would you use to achieve each of the...Ch. 9.6 - What reagents would you use to achieve each of the...Ch. 9.6 - What reagents would you use to achieve each of the...Ch. 9.6 - What reagents would you use to achieve each of the...Ch. 9.6 - What reagents would you use to achieve each of the...
Additional Science Textbook Solutions
Find more solutions based on key concepts
60. You are asked to consult for the city’s research hospital, where a group of doctors is investigating the bo...
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Consider the reaction: 2K3PO4(aq)+3NiCl2(aq)Ni3(PO4)2(s)+6KCl(aq) What volume of 0.225MK3PO4 solution is necess...
Introductory Chemistry (6th Edition)
Match each of the following items with all the terms it applies to:
Human Physiology: An Integrated Approach (8th Edition)
All of the following terms can appropriately describe humans except: a. primary consumer b. autotroph c. hetero...
Human Biology: Concepts and Current Issues (8th Edition)
Explain why 92% of 2,4-pemtanedione exists as the enol tautomer in hexane but only 15% of this compound exists ...
Organic Chemistry (8th Edition)
Which culture produces the most lactic acid? Use the following choices to answer questions. a. E. coli growing ...
Microbiology: An Introduction
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Basic strength of organic bases.arrow_forwardNucleophilic Aromatic Substitution: What is the product of the reaction? What is the name of the intermediate complex? *See imagearrow_forwardPredict the final product. If 2 products are made, list which should be “major” and “minor” *see attachedarrow_forward
- Nucleophilic Aromatic Substitution: What is the product of the reaction? *see imagearrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardThe answer here says that F and K have a singlet and a doublet. The singlet and doublet are referring to the H's 1 carbon away from the carbon attached to the OH. Why don't the H's two carbons away, the ones on the cyclohexane ring, cause more peaks on the signal?arrow_forward
- Draw the Birch Reduction for this aromatic compound and include electron withdrawing groups and electron donating groups. *See attachedarrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardBlocking Group are use to put 2 large sterically repulsive group ortho. Show the correct sequence toconnect the reagent to product with the highest yield possible. * see imagearrow_forward
- Elimination-Addition: What molecule was determined to be an intermediate based on a “trapping experiment”? *please solve and see imagearrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardPredict the final product. If 2 products are made, list which should be “major” and “minor”. **see attachedarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY