
Organic Chemistry As a Second Language: Second Semester Topics
4th Edition
ISBN: 9781119110651
Author: David R. Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9.6, Problem 9.33P
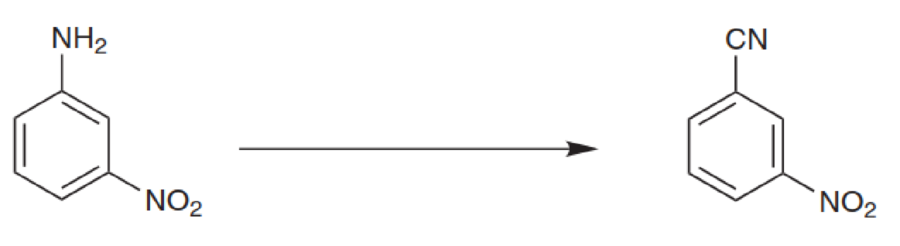
What reagents would you use to achieve each of the following transformations:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
7. Use Pauling's electronegativity values (Table 1.7) and Ketelaar triangle (Fig. 2.28) to classify
bonding in:
(3 points)
a) CIF3
b) ZnCl2
c) PbS
7. What is the IUPAC name of the following compound?
A) (R)-1-oxo-2-butanol
C) (R)-2-hydroxybutanal
E) (S)-1-formyl-1-propanol
B) (S)-1-oxo-2-butanol
D) (S)-2-hydroxybutanal
OH
H
Cual es la formula semidesarrollada del 3-metil-1-butino?
Chapter 9 Solutions
Organic Chemistry As a Second Language: Second Semester Topics
Ch. 9.2 - Identify how you would use a Gabriel synthesis to...Ch. 9.2 - Identify how you would use a Gabriel synthesis to...Ch. 9.2 - Identify how you would use a Gabriel synthesis to...Ch. 9.2 - Identify how you would use a Gabriel synthesis to...Ch. 9.2 - Identify whether each of the following compounds...Ch. 9.2 - Identify whether each of the following compounds...Ch. 9.2 - Identify whether each of the following compounds...Ch. 9.2 - Identify whether each of the following compounds...Ch. 9.3 - Suggest an efficient synthesis for each of the...Ch. 9.3 - Suggest an efficient synthesis for each of the...
Ch. 9.3 - Suggest an efficient synthesis for each of the...Ch. 9.3 - Suggest an efficient synthesis for each of the...Ch. 9.3 - Suggest an efficient synthesis for each of the...Ch. 9.3 - Prob. 9.18PCh. 9.3 - Prob. 9.19PCh. 9.3 - Prob. 9.20PCh. 9.3 - Prob. 9.21PCh. 9.3 - Prob. 9.22PCh. 9.4 - Prob. 9.24PCh. 9.4 - Prob. 9.25PCh. 9.5 - Predict the major product for each of the...Ch. 9.5 - Predict the major product for each of the...Ch. 9.5 - Predict the major product for each of the...Ch. 9.5 - Predict the major product for each of the...Ch. 9.6 - What reagents would you use to achieve each of the...Ch. 9.6 - What reagents would you use to achieve each of the...Ch. 9.6 - What reagents would you use to achieve each of the...Ch. 9.6 - What reagents would you use to achieve each of the...Ch. 9.6 - What reagents would you use to achieve each of the...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Suppose that in a lightning flash the potential difference between a cloud and the ground is 1.0 109 V and the...
Fundamentals of Physics Extended
explain the function of fermentation and the conditions under which it occurs?
Biology: Life on Earth with Physiology (11th Edition)
Two culture media were inoculated with four different bacteria. After incubation, the following results were ob...
Microbiology: An Introduction
8.63 Two flasks of equal volume and at the same temperature contain different gases. One flask contains 10.0 g ...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Which type of cartilage is most plentiful in the adult body?
Anatomy & Physiology (6th Edition)
Q3. An ion composed of which of these particles would have a mass of approximately 16 amu and a charge of 2−?
a...
Introductory Chemistry (6th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2. A graph shown below shows first ionization energies for elements from H to Ne. First ionization energy/kJ mol 2500 2000 1500 1000 500 T T T T 1 2 3 5 6 7 8 9 10 Atomic number a) Using arguments of electronic structure, explain why ionization energy of Li is much lower than that of H. (2 points) then dips at O. b) Using the same arguments, explain why ionization energy increases from B to N, and (3 points)arrow_forwardGive the name of this compound, including stereochemistry if relevant: CICH2 CH3 Br CH₂CH=CH2 Write in the product, including stereochemistry where relevant, for these reactions. See end of ch. 8, p. 301-303. 1. 03 a) 2-methyl-2-pentene -> 2. Zn, H* Br2 b) 1-ethylcyclopentene -->arrow_forwardNonearrow_forward
- 3. You may want to read paragraph 1.5 in your textbook before answering this question. Give electron configuration (short-hand notation is fine) for: (5 points) 3+ a) Manganese atom and Mn³+ b) Se atom c) Cu atom and Cu+arrow_forwardPlease correct answer and don't use hand ratingarrow_forwardNonearrow_forward
- Nonearrow_forwardHowever, why are intermolecular forces in metallic and ionic compounds not discussed as extensively? Additionally, what specific types of intermolecular attractions exist in metals and ionic compoundsarrow_forwardWhat is the preparation of 1 Liter of 0.1M NH4Cl buffer at pH 9.0 with solid NH4Cl and 0.1M NaOH. How would I calculate the math to describe this preparation? How would I use Henderson-Hasselbach equation?arrow_forward
- C Predict the major products of this organic reaction. Be sure you use wedge and dash bonds when necessary, for example to distinguish between major products with different stereochemistry. : ☐ + x G C RCO₂H Click and drag to start drawing a structure.arrow_forwardFill in the blanks by selecting the appropriate term from below: For a process that is non-spontaneous and that favors products at equilibrium, we know that a) ΔrG∘ΔrG∘ _________, b) ΔunivSΔunivS _________, c) ΔsysSΔsysS _________, and d) ΔrH∘ΔrH∘ _________.arrow_forwardHighest occupied molecular orbital Lowest unoccupied molecular orbital Label all nodes and regions of highest and lowest electron density for both orbitals.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY