
EBK ORGANIC CHEMISTRY
8th Edition
ISBN: 8220102744127
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 9.13, Problem 44P
(a)
Interpretation Introduction
Interpretation:
From the compounds given below one which is more reactive in SN1 reaction is to be identified.
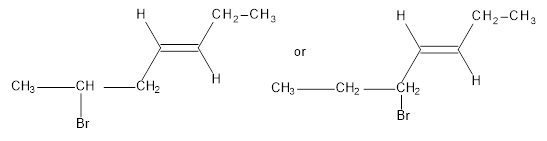
Concept Introduction:
If
(b)
Interpretation Introduction
Interpretation:
The product formed by SN1 reaction of the above compounds when ethanol is used as the solvent is to be identified.
Concept Introduction:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Find one pertinent analytical procedure for each of following questions relating to food safety analysis.
Question 1: The presence of lead, mercury and cadmium in canned tuna
Question 2: Correct use of food labelling
Formulate TWO key questions that are are specifically in relation to food safety.
In addition to this, convert these questions into a requirement for chemical analysis.
What are the retrosynthesis and forward synthesis of these reactions?
Chapter 9 Solutions
EBK ORGANIC CHEMISTRY
Ch. 9.1 - Prob. 3PCh. 9.1 - Does increasing the energy barrier for an SN2...Ch. 9.1 - Rank the following alkyl bromides from most...Ch. 9.1 - Prob. 8PCh. 9.2 - Prob. 9PCh. 9.2 - Prob. 10PCh. 9.2 - Prob. 11PCh. 9.2 - Prob. 12PCh. 9.2 - Which substitution reaction lakes place more...Ch. 9.2 - Prob. 15P
Ch. 9.2 - Prob. 17PCh. 9.3 - Prob. 18PCh. 9.4 - Prob. 19PCh. 9.5 - Draw the configuration(s) of the substitution...Ch. 9.5 - Which of the following reactions take place more...Ch. 9.7 - Prob. 22PCh. 9.7 - Prob. 23PCh. 9.7 - Prob. 24PCh. 9.7 - Prob. 25PCh. 9.8 - Four alkenes are formed from the E1 reaction of...Ch. 9.8 - If 2-fluoropentane could undergo an E1 reaction,...Ch. 9.8 - Prob. 28PCh. 9.8 - Propose a mechanism for the following reaction:Ch. 9.9 - Prob. 30PCh. 9.9 - What percentage of the reaction described in...Ch. 9.10 - Prob. 33PCh. 9.10 - Prob. 35PCh. 9.11 - Why do cis-1-bromo-2-ethylcyclohexane and...Ch. 9.11 - Which isomer reacts more rapidly in an E2...Ch. 9.11 - Prob. 38PCh. 9.12 - Prob. 39PCh. 9.12 - Prob. 40PCh. 9.12 - Prob. 41PCh. 9.12 - Explain why only a substitution product and no...Ch. 9.12 - a. Explain why 1-bromo-2,2-dimethylpropane has...Ch. 9.13 - Prob. 44PCh. 9.13 - Prob. 45PCh. 9.13 - What products will be obtained from the El...Ch. 9.13 - Prob. 47PCh. 9.13 - Prob. 48PCh. 9.13 - Prob. 49PCh. 9.13 - Why is a cumulated diene not formed in the...Ch. 9.13 - What product is obtained when the following...Ch. 9.13 - What products are formed from the following...Ch. 9.14 - Prob. 54PCh. 9.14 - Prob. 55PCh. 9.14 - Prob. 56PCh. 9.14 - Prob. 58PCh. 9.14 - Under which of the following reaction conditions...Ch. 9.15 - A small amount of another organic product is...Ch. 9.15 - What is the best way to prepare the following...Ch. 9.15 - Prob. 62PCh. 9.15 - What products (including stereoisomers, if...Ch. 9.16 - After a proton is removed from the OH group, which...Ch. 9.16 - Prob. 65PCh. 9.17 - Prob. 66PCh. 9 - Prob. 1PCh. 9 - Methoxychlor is an insecticide that was intended...Ch. 9 - Prob. 67PCh. 9 - Prob. 68PCh. 9 - Prob. 69PCh. 9 - Prob. 70PCh. 9 - Prob. 71PCh. 9 - Prob. 72PCh. 9 - Starting with cyclohexene, how can the following...Ch. 9 - Prob. 74PCh. 9 - The pKa of acetic acid in water is 4.76. What...Ch. 9 - Prob. 76PCh. 9 - Prob. 77PCh. 9 - Prob. 78PCh. 9 - Prob. 79PCh. 9 - Prob. 80PCh. 9 - Prob. 81PCh. 9 - Prob. 82PCh. 9 - Draw the major product obtained when each of the...Ch. 9 - Prob. 84PCh. 9 - a. Indicate how each of the following factors...Ch. 9 - Prob. 86PCh. 9 - A chemist wanted to synthesize the...Ch. 9 - Prob. 88PCh. 9 - Prob. 89PCh. 9 - Prob. 90PCh. 9 - Prob. 91PCh. 9 - Starting with an alkyl halide, how could the...Ch. 9 - Indicate which species in each pair gives a higher...Ch. 9 - Prob. 94PCh. 9 - Rank the following from most reactive to least...Ch. 9 - For each of the following alkyl halides, indicate...Ch. 9 - Prob. 97PCh. 9 - When 2-bromo-2,3-dimethylbutane reacts with a...Ch. 9 - Prob. 100PCh. 9 - When the following compound undergoes solvolysis...Ch. 9 - cis-1-Bromo-4-tert-butylcyclohexane and...Ch. 9 - Draw the substitution and elimination products.Ch. 9 - tert-Butyl chloride undergoes solvolysis in both...Ch. 9 - Prob. 105PCh. 9 - Prob. 106PCh. 9 - In which solventethanol or diethyl etherwould the...Ch. 9 - Prob. 108PCh. 9 - Two bromoethers are obtained from the reaction of...Ch. 9 - Prob. 110PCh. 9 - Prob. 111PCh. 9 - Prob. 112PCh. 9 - Which of the following hexachlorocyclohexanes is...Ch. 9 - Explain why the rate of the reaction of...Ch. 9 - Prob. 115PCh. 9 - Two elimination products are obtained from the...Ch. 9 - Draw the structures or the product of the obtained...Ch. 9 - How could you prepare the following compounds from...Ch. 9 - cis-4-Bromocyclohexanol and...Ch. 9 - Prob. 120PCh. 9 - Propose a mechanism for the following reaction:Ch. 9 - Prob. 122PCh. 9 - Prob. 123PCh. 9 - Prob. 124PCh. 9 - Prob. 125PCh. 9 - Predict the product for the following reaction and...Ch. 9 - Prob. 127PCh. 9 - Prob. 128PCh. 9 - When equivalent amounts of methyl bromide nod...Ch. 9 - Prob. 130PCh. 9 - The reaction of chloromethane with hydroxide ion...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the given reactions would form meso product? H₂O, H2SO4 III m CH3 CH₂ONa CH3OH || H₂O, H2SO4 CH3 1. LiAlH4, THF 2. H₂O CH3 IVarrow_forwardWhat is the major product of the following reaction? O IV III HCI D = III ა IVarrow_forwardThe reaction of what nucleophile and substrate is represented by the following transition state? CH3 CH3O -Br อ δ CH3 Methanol with 2-bromopropane Methanol with 1-bromopropane Methoxide with 1-bromopropane Methoxide with 2-bromopropanearrow_forward
- What is the stepwise mechanism for this reaction?arrow_forward32. Consider a two-state system in which the low energy level is 300 J mol 1 and the higher energy level is 800 J mol 1, and the temperature is 300 K. Find the population of each level. Hint: Pay attention to your units. A. What is the partition function for this system? B. What are the populations of each level? Now instead, consider a system with energy levels of 0 J mol C. Now what is the partition function? D. And what are the populations of the two levels? E. Finally, repeat the second calculation at 500 K. and 500 J mol 1 at 300 K. F. What do you notice about the populations as you increase the temperature? At what temperature would you expect the states to have equal populations?arrow_forward30. We will derive the forms of the molecular partition functions for atoms and molecules shortly in class, but the partition function that describes the translational and rotational motion of a homonuclear diatomic molecule is given by Itrans (V,T) = = 2πmkBT h² V grot (T) 4π²IKBT h² Where h is Planck's constant and I is molecular moment of inertia. The overall partition function is qmolec Qtrans qrot. Find the energy, enthalpy, entropy, and Helmholtz free energy for the translational and rotational modes of 1 mole of oxygen molecules and 1 mole of iodine molecules at 50 K and at 300 K and with a volume of 1 m³. Here is some useful data: Moment of inertia: I2 I 7.46 x 10- 45 kg m² 2 O2 I 1.91 x 101 -46 kg m²arrow_forward
- K for each reaction step. Be sure to account for all bond-breaking and bond-making steps. HI HaC Drawing Arrows! H3C OCH3 H 4 59°F Mostly sunny H CH3 HO O CH3 'C' CH3 Select to Add Arrows CH3 1 L H&C. OCH3 H H H H Select to Add Arrows Q Search Problem 30 of 20 H. H3C + :0: H CH3 CH3 20 H2C Undo Reset Done DELLarrow_forwardDraw the principal organic product of the following reaction.arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided structures, draw the curved arrows that epict the mechanistic steps for the proton transfer between a hydronium ion and a pi bond. Draw any missing organic structures in the empty boxes. Be sure to account for all lone-pairs and charges as well as bond-breaking and bond-making steps. 2 56°F Mostly cloudy F1 Drawing Arrows > Q Search F2 F3 F4 ▷11 H. H : CI: H + Undo Reset Done DELLarrow_forward
- Calculate the chemical shifts in 13C and 1H NMR for 4-chloropropiophenone ? Write structure and label hydrogens and carbons. Draw out the benzene ring structure when doing itarrow_forward1) Calculate the longest and shortest wavelengths in the Lyman and Paschen series. 2) Calculate the ionization energy of He* and L2+ ions in their ground states. 3) Calculate the kinetic energy of the electron emitted upon irradiation of a H-atom in ground state by a 50-nm radiation.arrow_forwardCalculate the ionization energy of He+ and Li²+ ions in their ground states. Thannnxxxxx sirrr Ahehehehehejh27278283-4;*; shebehebbw $+$;$-;$-28283773838 hahhehdvaarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning