
The wastewater treatment plant at the Ossabaw Paper Company paper mill generates about 24 tonnes of sludge per day. The consistency of the sludge is 35%, meaning that the sludge contains 35 wt% solids and the balance liquids. The mill currently spends $40/tonne to dispose of the sludge in a landfill. The plant environmental engineer has determined that if the sludge consistency could be increased to 75%, the sludge could be incinerated (burned) to generate useful energy and to eliminate the environmental problems associated with landfill disposal.
A flowchart for a preliminary design of the proposed sludge-treatment process follows. For simplicity, we will assume that the liquid in the sludge is just water.
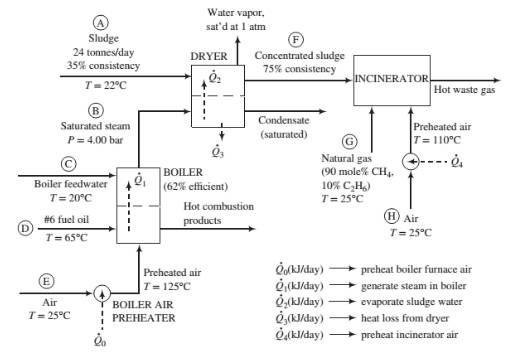 Process description: The sludge from the wastewater treatment plant (Stream (A) passes through a dryer where a portion of the water in the sludge is vaporized. The heat required for the vaporization comes from condensing saturated steam at 4.00 bar (Stream (B)). The steam fed to the dryer is produced in the plant’s oil-fired boiler from feedwater at 20°C (Stream (C)). The heat required to produce the steam is transferred from the boiler furnace, where fuel oil (Stream (D)) is burned with 25% excess air (Stream (E)). The concentrated sludge coming from the dryer (Stream (F)), which has a consistency of 75%, is fed to an incinerator. The heating value of the sludge is insufficient to keep the incinerator temperature high enough for complete combustion, so natural gas (Stream (G)) is used as a supplementary fuel. A stream of outside air at 25°C (Stream (H)) is heated to 110°C and fed to the incinerator along with the concentrated sludge and natural gas. The waste gas from the incinerator is discharged to the atmosphere.
Process description: The sludge from the wastewater treatment plant (Stream (A) passes through a dryer where a portion of the water in the sludge is vaporized. The heat required for the vaporization comes from condensing saturated steam at 4.00 bar (Stream (B)). The steam fed to the dryer is produced in the plant’s oil-fired boiler from feedwater at 20°C (Stream (C)). The heat required to produce the steam is transferred from the boiler furnace, where fuel oil (Stream (D)) is burned with 25% excess air (Stream (E)). The concentrated sludge coming from the dryer (Stream (F)), which has a consistency of 75%, is fed to an incinerator. The heating value of the sludge is insufficient to keep the incinerator temperature high enough for complete combustion, so natural gas (Stream (G)) is used as a supplementary fuel. A stream of outside air at 25°C (Stream (H)) is heated to 110°C and fed to the incinerator along with the concentrated sludge and natural gas. The waste gas from the incinerator is discharged to the atmosphere.
Fuel oil: The oil is a low-sulfur No. 6 fuel oil. Its ultimate (elemental) analysis on a weight basis is 87% C, 10% H, 0.84% S, and the balance oxygen, nitrogen, and nonvolatile ash. The higher heating value of the oil is 3.75 × 104kJ/kg and the heat capacity is Cp= 1.8 kJ/(kg·°C).
Boiler: The boiler has an efficiency of 62%, meaning that 62% of the heating value of the fuel oil burned is used to produce saturated steam at 4.00 bar from boiler feedwater at 20°C. Fuel oil at 65°C and dry air at 125°C are fed to the boiler furnace. The air feed rate is 25% in excess of the amount theoretically required for complete consumption of the fuel.
Sludge: The sludge from the wastewater treatment plant contains 35% w/w solids (S) and the balance liquids (which for the purposes of this problem may be treated as only water) and enters the dryer at 22°C. The sludge includes a number of volatile organic species, some of which may be toxic, and has a terrible odor. The heat capacity of the solids is approximately constant at 2.5 kJ/(kg·°C).
Dryer: The dryer has an efficiency of 55%, meaning that the heat transferred to the sludge, 02, is 55% of the total heat lost by the condensing steam, and the remainder,
Incinerator: The concentrated sludge has a heating value of 19,000 kJ/kg dry solids. For a feed sludge of 75% consistency, the incinerator requires 195 SCM natural gas/tonne wet sludge [ 1 SCM = 1 m3(STP)]. The theoretical air requirement for the sludge is 2.5 SCM air/10.000 kJ of heating value. Air is fed in 100% excess of the amount theoretically required to bum the sludge and the natural gas.
Use material and energy balances to calculate the mass flow rates (tonnes/day) of Streams (B), (C), (D), (E), (F), (G) and (H), and heat flows
- The money saved by implementing this process will be the current cost of disposing of the wastewater plant sludge in a landfill. Two major costs of implementing the process are the installed costs of the new dryer and incinerator. What other costs must be taken into account when determining the economic feasibility of the process? Why might management decide to go ahead with the project even if it proves to be unprofitable?
- What opportunities exist for improving the energy economy of the process? (Hint: Think about the need to preheat the fuel oil and the boiler and incinerator air streams and consider heat exchange possibilities.)
- The driving force for the introduction of this process is to eliminate the environmental cost of sludge disposal. What is that cost—that is, what environmental penalties and risks are associated with using landfills for hazardous waste disposal? What environmental problems might incineration introduce?
Learn your wayIncludes step-by-step video

Chapter 9 Solutions
ELEMENTARY PRINCIPLES OF CHEM. PROCESS.
Additional Science Textbook Solutions
Problem Solving with C++ (10th Edition)
Java: An Introduction to Problem Solving and Programming (8th Edition)
Concepts Of Programming Languages
Management Information Systems: Managing The Digital Firm (16th Edition)
Database Concepts (8th Edition)
Web Development and Design Foundations with HTML5 (8th Edition)
- Q1: Answer the questions for the reaction below: ..!! Br OH a) Predict the product(s) of the reaction. b) Is the substrate optically active? Are the product(s) optically active as a mix? c) Draw the curved arrow mechanism for the reaction. d) What happens to the SN1 reaction rate in each of these instances: 1. Change the substrate to Br "CI 2. Change the substrate to 3. Change the solvent from 100% CH3CH2OH to 10% CH3CH2OH + 90% DMF 4. Increase the substrate concentration by 3-fold.arrow_forwardExperiment 27 hates & Mechanisms of Reations Method I visual Clock Reaction A. Concentration effects on reaction Rates Iodine Run [I] mol/L [S₂082] | Time mo/L (SCC) 0.04 54.7 Log 1/ Time Temp Log [ ] 13,20] (time) / [I] 199 20.06 23.0 30.04 0.04 0.04 80.0 22.8 45 40.02 0.04 79.0 21.6 50.08 0.03 51.0 22.4 60-080-02 95.0 23.4 7 0.08 0-01 1970 23.4 8 0.08 0.04 16.1 22.6arrow_forward(15 pts) Consider the molecule B2H6. Generate a molecular orbital diagram but this time using a different approach that draws on your knowledge and ability to put concepts together. First use VSEPR or some other method to make sure you know the ground state structure of the molecule. Next, generate an MO diagram for BH2. Sketch the highest occupied and lowest unoccupied MOs of the BH2 fragment. These are called frontier orbitals. Now use these frontier orbitals as your basis set for producing LGO's for B2H6. Since the BH2 frontier orbitals become the LGOS, you will have to think about what is in the middle of the molecule and treat its basis as well. Do you arrive at the same qualitative MO diagram as is discussed in the book? Sketch the new highest occupied and lowest unoccupied MOs for the molecule (B2H6).arrow_forward
- Q8: Propose an efficient synthesis of cyclopentene from cyclopentane.arrow_forwardQ7: Use compound A-D, design two different ways to synthesize E. Which way is preferred? Please explain. CH3I ONa NaOCH 3 A B C D E OCH3arrow_forwardPredict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2).arrow_forward
- (10 pts) The density of metallic copper is 8.92 g cm³. The structure of this metal is cubic close-packed. What is the atomic radius of copper in copper metal?arrow_forwardPredict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2).arrow_forwardPredict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2).arrow_forward
- Q3: Rank the following compounds in increasing reactivity of E1 and E2 eliminations, respectively. Br ca. go do A CI CI B C CI Darrow_forwardQ5: Predict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2). H₂O דיי "Br KN3 CH3CH2OH NaNH2 NH3 Page 3 of 6 Chem 0310 Organic Chemistry 1 HW Problem Sets CI Br excess NaOCH 3 CH3OH Br KOC(CH3)3 DuckDuckGarrow_forwardQ4: Circle the substrate that gives a single alkene product in a E2 elimination. CI CI Br Brarrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
