
Concept explainers
Ethylbenzene is converted to styrene in the catalytic dehydrogenation reaction C«H10(g) -
A flowchart of a simplified version of the commercial process is shown here.
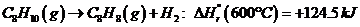
Fresh and recycled liquid ethylbenzene combine and are heated from 25°C to 500°C (A). and the heated ethylbenzene is mixed adiabatically with steam at 700°C (B) to produce the feed to the reactor at 600°C. (The steam suppresses undesired side reactions and removes carbon deposited on the catalyst surface.) A once-through conversion of 35% is achieved in the reactor (C), and the products emerge at 560°C. The product stream is cooled to 25°C (D), condensing essentially all of the water, ethylbenzene, and styrene and allowing hydrogen to pass out as a recoverable by-product of the process.
The water and hydrocarbon liquids are immiscible and are separated in a settling tank decanter (£). The water is vaporized and healed (F) to produce the steam that mixes with the ethylbenzene feed to the reactor. The hydrocarbon stream leaving the decanter is fed to a distillation tower(C) (actually, a series of towers), which separates the mixture into essentially pure styrene and ethylbenzene, each at 25°C after cooling and condensation steps have been carried out. The ethylbenzene is recycled to the reactor preheater, and the styrene is taken off as a product.
- On a basis of 100 kg/h styrene produced, calculate the required fresh ethylbenzene feed rale, the flow rate of recycled ethylbenzene, and the circulation rate of water, all in mol/h. (Assume P = 1 atm.)
- Calculate the required rates of heat input or withdrawal in kJ/h for the ethylbenzene preheater (A), steam generator (F), and reactor (C).
Physical Property Data
Learn your wayIncludes step-by-step video

Chapter 9 Solutions
ELEMENTARY PRINCIPLES OF CHEM. PROCESS.
Additional Science Textbook Solutions
Elementary Surveying: An Introduction To Geomatics (15th Edition)
Electric Circuits. (11th Edition)
Database Concepts (8th Edition)
Modern Database Management
Mechanics of Materials (10th Edition)