
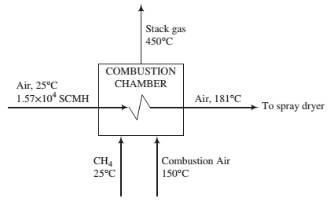
Methane is burned completely with 40% excess air. The methane enters the combustion chamber at 25°C, the combustion air enters at 150°C. and the stack gas [CO2, H2O(v), O2, N2] exits at 450°C. The chamber functions as a preheater for an air stream flowing in a pipe through the chamber to a spray dryer. The air enters the chamber at 25°C at a rate of 1.57 × 104m3(STP)/h and is heated to 181°C. All of the heat generated by combustion is used to heat the combustion products and the air going to the spray dryer (i.e., the combustion chamber may be considered adiabatic).
- Draw and completely label the process flow' diagram and perform a degree-of-freedom analysis.
Want to see the full answer?
Check out a sample textbook solution
Chapter 9 Solutions
ELEMENTARY PRINCIPLES OF CHEM. PROCESS.
Additional Science Textbook Solutions
Starting Out With Visual Basic (8th Edition)
Concepts Of Programming Languages
Starting Out with Python (4th Edition)
SURVEY OF OPERATING SYSTEMS
Computer Science: An Overview (13th Edition) (What's New in Computer Science)
Starting Out with Java: From Control Structures through Data Structures (4th Edition) (What's New in Computer Science)
- A fluidized bed reactor uses a solid catalyst with a particle diameter of 0.25 mm, a bulk density of 1.50 g/mL, and a sphericity of 0.90. Under packed bed conditions, the porosity is 0.35, and the bed height is 2 m. The gas enters from the bottom of the reactor at a temperature of 600°C, with a viscosity of 0.025 cP and a density of 0.22 lb/ft³. At minimum fluidization, the porosity reaches 0.45. Calculate: a. The minimum superficial velocity (VM) of the gas entering the fluidized column. b. The bed height if V = 2 VM c. The pressure drop under conditions where V =2.5 VMarrow_forwardPlease answer 5.8arrow_forwardPlease answer 5.6arrow_forward
- You have been tasked with figuring out how to suppress changes in the supply flow rate to a reactorfor which it is desired to keep the inlet flow rate as constant as possible. You are considering designing a surgetank to place upstream of the reactor and then installing a pump on the line between that surge tank and thereactor. A surge tank is one with a weir inside it, which is a partial wall separating the tank volume into twoconnected sections allowing for flow under the weir between the two sections. The variable inlet mass flow,wi(t) flows into volume 1 and then flows due to hydrostatic pressure at a mass flow rate of w1(t) into volume 2.The weir causes a flow resistance, R1, such that w1 = (h1-h2)/R1. Fluid is then pumped out of volume 2 at thedesired constant mass flow rate of w2. Make a summary table of the three transfer functions written in standard form and their keyparameters (gains, time constants) in terms of the physical system parameters (A1, A2, , A, R…). Checkif/how…arrow_forwardThe vapor pressure of Toluene at 50°C in Pa? Find it on perry's chemical engineering handbook 9th or 8th editionarrow_forwardHydrogen (H₂) is considered a clean energy carrier. For its use as a fuel, hydrogen is stored at 5 bar insidea cylindrical tank made of nickel (Ni) with 7 cm inner diameter, 1.2 mm thickness, and the length of L. Thetank is maintained at 358 K. Unfortunately, a small amount of hydrogen diffuses out of the tank, slowlydepleting its contents. You may assume that the hydrogen pressure outside the tank is essentially zero andconvective resistance inside and outside of the cylinder is negligible.• Solubility of H2 in Ni at 358 K = 0.00901 kmol/m3·bar• DH2, Ni at 358 K = 1.2 x 10-12 m2/sCalculate the maximum length of the nickel tank wall to ensure that the hydrogen loss does not exceed0.01 kg per year.arrow_forward
- You just took out a cold soda can (at 1 oC) from the refrigerator. Calculate thetemperature of the soda can after the can is placed in a room (at 31 oC, h = 100 W/m2-K) for 60 mins (we all know that soda tastes much better when it is cold!). • k = 0.617 W/m-K, density = 996 kg/m3, Cp = 4178 J/Kg-K• Height = 10 cm & Diameter = 5 cmCalculate the temperature of the soda can surface at the middle point of the heightusing 2-D analysis.arrow_forwardA thick nickel wall is exposed to pure 5 bar H2(g) at 85 oC on one side of its surface (13 pts).(a) Assuming thermodynamic gas-solid equilibrium, calculate the H2 concentration at the surface ofthe nickel wall. (b) Assuming that the concentration of H2 at the surface is constant, determine the concentration ofH2 at the penetration depth in percentage of its concentration at the wall surfacearrow_forwardCan you provide me the answer of these pleasearrow_forward
- A constant-volume process involving 2.0 moles of diatomic ideal gas, for which cV = (5/2)R, is at an initial state of 111 kPa and 277 K and is then heated reversibly to 356 K. Calculate the P2 (kPa), dU (kJ), heat transfer q (kJ mol–1), and w (kJ mol–1).arrow_forwardProblem 1 Marks: 60 Section: 1a): 30 marks, Section 1b):30 marks A laboratory scale fluidized bed is considered for studying a catalytic ozone decomposition. a) It is requested to derive model equations under the following assumptions: ■ Operation of the catalytic reactor under steady state conditions, There is no influence of thermal ozone decomposition reactions. The fluidized bed includes bubbles and dense phase. □ The dense phase can be simulated using a CSTR The fluidized bed bubbles contain catalyst particles and can be simulated as a DSTR (batch). □ The jets contain particles and can be simulated with a PFR. The influence of the freeboard has to be considered using a PFR model. The available catalytic reaction rate model is r (moles/gcat.s)= -k CA b) Same than on a) under unsteady state conditions, using an absorbable and reactive tracer. Note: A step-by step derivation of the model equations is required here. A quick answer will not do. Problem 2 Marks: 40 Section 2a: 30 marks,…arrow_forwardPenicillin process Penicillium chrysogenum is used to produce penicillin in a 90,000-litre fermenter. The volumetric rate of oxygen uptake by the cells ranges from 0.45 to 0.85 mmol l^−1 min^−1 depending on time during the culture. Power input by stirring is 2.9 W l^−1. Estimate the cooling requirements.arrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





