
Concept explainers
(a)
Interpretation:
Taking a basis of 1 mol of feed gas, the moles of each component of the feed product mixture and extent of reaction should be calculated.
Concept Introduction
The flow chart for the given situation would be helpful to solve the problem.
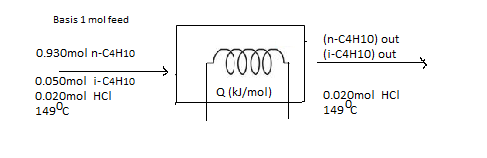
(b)
Interpretation:
First, the calculation of the standard heat of the isomerization reaction (kJ) should be found. Then, taking the feed and product species at 25? as references, an inlet-outlet enthalpy table should be prepared and component amounts (mol) and specific enthalpies (kJ/mol) should be calculated and filled in.
Concept introduction:
The standard heat of formation will be helpful to solve the problem as:
(c)
Interpretation:
The amount of heat transferred should be calculated in kJ and the required heat transfer for a reactor feed of 325 kJ/h should be determined in kW.
Concept introduction:
The heat transferred is calculated using formula:
(d)
Interpretation:
The calculated results should be used to estimate the heat of the isomerization reaction at 149? and the assumptions built into the estimation should be listed.
Want to see the full answer?
Check out a sample textbook solution
Chapter 9 Solutions
ELEMENTARY PRINCIPLES OF CHEM. PROCESS.
- heat and mass transferarrow_forwardSteam at atmospheric pressure (Tsat = 100oC, hfg = 2.257×106 J/kg) is in contact with a horizontal tube through which a cooling fluid is circulated. The tube has 0.0334 m outside diameter, 1 m length, and an outside-surface temperature that is maintained at 60oC. Determine the rate of heat that the cooling fluid must remove and the condensation rate. For water at average temperature, ρ L = 974 kg/m3, k = 0.668 W/m·K, ρ v = 0.516 kg/m3, ν L = 0.364×10-6m2/s. g =9.81 m/s2.arrow_forwardSurface A1 of the system shown in the figure below is a graybody with emissivity of 0.56 andsurface A2 is a blackbody.Can you determine view factors F1-2 and F2-1. And draw an analogous electrical circuit based on Ohm’s law and determine the net radiation heat transfer from surface A1 to surface A2 if T1 = 500oC and T2 = 27oC. For the graybody, α = ε. Stefan-Boltzmann constant, σ = 5.676 × 10-8 W/m2·K4.arrow_forward
- An aluminum saucepan has a handle that is fixed to its wall. The handle itself is made of low carbon steel, and will have a plastic grip attached to it that is comfortable to grasp. Before selecting a plastic, it is necessary to have information on the temperature of the carbon steel handle. The carbon steel handle can be considered as a rod 11 mm in diameter and 45 mm long. When being used over a stove burner, the ambient temperature T∞ is 30 oC, and the temperature at the base of the handle reaches T0 = 100 oC. The convective heat transfer coefficient h is 8 W/m2·K and k = 43 W/m·K for low carbon steel. Can you derive the differential equation for the temperature of handle with x as a spatial variable and determine the temperature of handle at the position of 40 mm from the base. Using the general solution attached. Also Can you determine the total heat transfer rate (q) from the handle using the above temperature profile equation and determine the total heat transfer rate (q) from…arrow_forwardFor forced convection, the important variables, their symbols, and dimensional representations are listed below. From the Buckingham method of grouping the variables, the rank of the dimensional matrix is 4. What is the number of independent dimensionless groups in this case? By utilizing the dimensional- analysis approach, find a dimensionless group. (Show just one dimensionless group you can find)arrow_forwardA parallel flow heat exchanger with surface area of 62.5 m2 is to be designed for cooling oil at 80oC with specific heat of 2.5 kJ/kg·oC and mass flow rate of 5.0 kg/s. Water at 15oC flowing at 9.94 kg/s is used as the cooling fluid (cp = 4.19 kJ/kg·oC). 1 W = 1 J/s Can you determine the heat transfer rate and outlet temperatures, assuming an overall heat transfer coefficient equal to 300 W/m2·oC. For heat transfer rate, use the effectiveness, ε, and Check the results with log-mean temperature difference approach. (Calculate the heat transfer rate and compare the value in (1))varrow_forward
- A 5 cm diameter, 60 cm long aluminum cylinder initially at 50 oC is submerged in an ice-water bath at 2 oC. The convective heat transfer coefficient between the metal and the bath is 550 W/m2·K. Using the lumped parameter analysis, derive the equation below. Show procedures in detail please.And determine the central temperature of the aluminum after 1 min. For aluminum, k = 236 W/m·K, ρ =2702 kg/m3, and cp= 896 J/kg·K. (1 W = 1 J/s) Then calculate the cumulative heat transfer for the first 1 min.(the first law of theromodynamics is attached and the. needed equation to derive)arrow_forwardRadioactive waste is stored in the spherical stainless-steel container as shown schematically in the figure below. Heat generated within the waste material must be dissipated by conduction through the steel shell and then by convection to the cooling water. Pertinent dimensions and system operating variables are Inside radius of steel shell, ri = 0.4 m; Outside radius of steel shell, ro = 0.5 m Volumetric rate of heat generated within waste material, q˙= 105 W/m3, Thermal conductivity of steel = 15 W/m·K; Thermal conductivity of waste = 20 W/m·K, Convective heat-transfer coefficient between steel shell and water, h = 800 W/m2·K, Water temperature, T∞ = 25 oC. In the stainless-steel shell, the temperature profile as a function of radius, r can be expressed as follows:Can you: At steady state, calculate the temperature at the outside steel surface (r = ro). And Calculate the temperature at the inside steel surface (r = ri).arrow_forwardcan you do specific control loop for dc including reboiler and condenserarrow_forward
- The periodic array below denotes a hypothetical lattice in 2D. 1. Is this a Bravais lattice? Why or why not? 2. Draw 3 different versions of unit cells. Of those 3, make one a primitive unit cell. 3. There is a special type of primitive unit cell that is called a ’Wigner-Seitz’ cell (look it up on- line, or in text books). In words, describe how it is defined, and draw it for the lattice above. 4. Why would it be useful to draw the primitive unit cell of a lattice as a Wigner-Seitz cell, rather than another random choice? Hint : What geometric property of a lattice was described to be useful in guessing material propertiesarrow_forwardThe schematic figure below shows a face-centered cubic (FCC) lattice with a lattice constant of a. Draw the lattice vectors of a primitive unit cell for the FCC lattice. Also write them in equation form and derive an expression for the reciprocal lattice vectors of an FCC lattice Using these sets of reciprocal lattice vectors, draw (or generate) the reciprocal lattice of FCC in re- ciprocal space. Show that this reciprocal lattice is equivalent to a body-centered cubic lattice with a lattice constant of 4pi/aarrow_forwardDerive the 3D density of states for a system of electrons with the dispersion relation (in the picture) Assume that there is no spin degeneracyarrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





