
Assume that two liquids are separated by a semipermeable membrane, with pure solvent on the right side and a solution of a solute on the left side. Make a drawing that shows the situation after equilibrium is reached.
Interpretation:
The situation after equilibrium is reached when two liquids are separated by a semipermeable membrane has to be drawn.
Concept introduction:
Colligative properties such as vapor pressure, freezing point, and boiling point are affected by the presence of solute particles in a solution.
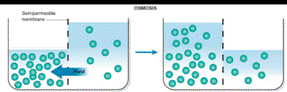
Osmosis is the passage of substances in true solution through a semipermeable membrane.
Particles such as starches and protein molecules are too large to pass the membrane.
The restriction of passage of large particles causes the smaller particles to pass more rapidly in the direction of higher concentrations, producing an osmotic pressure in confined liquids.
Explanation of Solution
Osmosis occurs when solution of different concentration are separated by a semipermeable membrane that allows solvent molecules to pass but blocks the passage of solute ions and molecules. Solvent flows from the more dilute side to the more concentrated side until sufficient osmotic pressure builds up and stops the flows. An effect similar to osmosis occurs when membranes of large pore size are used.
The situation after equilibrium is reached when two liquids are separated by a semipermeable membrane is drawn as
Want to see more full solutions like this?
Chapter 9 Solutions
FUND.OF GEN CHEM CHAP 1-13 W/ACCESS
- Determine Km and Vmax from the michaelis menten grapharrow_forwardDetermine the Km and Vmax from the lineweuver burk grapharrow_forwardDo schwann cells produce or act as myelin in the peripheral nervous system? I know that they encase and wrap around axons, but where does the myelin come into play?arrow_forward
- The enzyme lactate dehydrogenase (LDH) catalyzes the conversion of pyruvate to lactatein skeletal muscle cells using NAD/NADH during anaerobic “balanced” fermentation.Answer the following questions about this reaction. (a) Write out the two reductive half reactions and indicate the E ̊' for each half reaction. Write out the full balanced reaction for the pyruvate to lactate rxn and indicate the ∆E ̊' for the reaction. (b) What is the free energy change under standard state conditions for thisreaction? Which direction is spontaneous?(c) Assume that in skeletal muscle cells the ratio of [NAD+] to [NADH] is 100, and that the[pyruvate] = 0.40 mM and [lactate] = 4.0 mM. What is the free energy change (∆G')for the conversion of pyruvate to lactate? Indicate the direction in which the reactionis spontaneous under these cellular conditions.arrow_forwardWhy did the authors worry about the temperature-dependent solubility of the carriers in thebilayer? How did the authors determine whether the effect of freezing the lipid bilayer wasto decrease the solubility of the carriers (nonactin and valinomycin) or whether the effectwas to impair their ability to diffuse through the membrane (decrease their mobility)?arrow_forwardKranse et. al. measured the temperature dependence of conductance using membranescontaining the phospholipids glyceryl dipalmitate and glyceryl distearate. Describe themodifications in membrane content that you would employ to: (a) shift the temperature of the phase transition (b) make the ion conductance curve for valinomycin andnonactin more like that of gramicidinarrow_forward
- Obtain the sequence for the 5-HT receptor HTR1A and generate a hydropathy plot usingthe ExPASY tool ProtScale, the appropriate window, and the Kyte-Doolittle weightingalgorithm. How many transmembrane domains are present in this receptor? Attach yourhydropathy plot to your assignment.arrow_forwardCompare and contrast the structural features of the ion carrier valinomycin with those of achannel former like gramicidin. How does structural information help explain the mechanismby which these molecules conduct ions across membranes?arrow_forwardA typical integral membrane protein has a stretch (or stretches) of ~20 hydrophobic aminoacids that form an α-helix that spans the bilayer (as is found in membrane proteins such asglycophorin A and bacteriorhodopsin). Compare and contrast the molecular and structural features of gramicidin with a membrane-spanning α-helix. Explain how gramicidin can forman ion channel when a typical membrane-spanning α-helix cannot (eg, glycophorin A).arrow_forward
- The titration curve of alanine shows the ionization of two functional groups with pK values of 2.34 and 9.69, corresponding to the ionization of the carboxyl and the protonated amino groups, respectively. The titration of di-, tri-, and larger oligopeptides of alanine also shows the ionization of only two functional groups, although the experimental pK values are different. The table summarizes the trend in pK values. Amino acid or peptide Ala Ala-Ala pKj pk₂ 2.34 9.69 3.12 8.30 Ala-Ala-Ala 3.39 8.03 Ala-(Ala)-Ala, n ≥ 4 3.42 7.94 Modify the molecules to show the oligopeptide Ala-Ala-Ala. You can modify the molecules by moving, adding, deleting, or changing atoms, bonds, or charges. C Select c Draw Templates More H с N 0 S Cl H H | | || H CH3 H CH, H CH₂ Complete the statements about the the pK, values of the Ala-Ala-Ala oligopeptide. The pK₁ value of 3.39 is associated with the -COO group of Ala-Ala-Ala. The pK2 value of 8.03 is associated with the -NH group of Ala-Ala-Ala. Erase Q2 Q…arrow_forwardFacts from the bacterium mals and to dept kan apa in a peptide with antidic properties. This peptide complex with the call membrance of other hacterial species, leading in bacterial death The structure of the peptide has been determined from (a) Cmplete acid hydes of the peptide, followed by amino acid analys, yielded quiar anunt of Lan, Om, Pfx, Prxa, and Wall Cmtiti, an amino acid od prosentin pockets but present in some peptides. Com has the tracture H *H,N-CH-CH-CH, -C- COO (b) The weight of the peptide in approximately 1,200 Th (c) The peptide failed to undergo hydrolysis when treated with the Hydrolysis of the carbonyl-terminal residue of a polypeptide une "NH, the year. This call there Pro or the police does not contain a froz (d) Treatment of the peptide with 1-haw-2,4-dicherer (11N1), followed by complete hydrolysis and ched only from and the derivative NO, Н ON NHCHI CH, CH, C coo +NH, (Hint: The 2,4-diphenyl derivative involves the amino group of a side chain rather than the…arrow_forwardElectrophoresis Macmillan Learning Chymotrypsin is a protease with a molecular mass of 25.6 kDa. The figure shows a stained SDS polyacrylamide gel with a single band in lane I and three bands of lower molecular weight in lane 2. Lane I contains a preparation of chymotrypsin and lane 2 contains chymotrypsin pre-treated with performic acid. 1 2 Why does performic acid treatment of chymotrypsin generate three bands in lane 2? ° Chymotrypsin self-digests on the carboxyl-terminal side of phenylalanine, tryptophan, or tyrosine residues. The three peptides are impurities in the original chymotrypsin sample. Performic acid cleaves proteins on the carboxyl-terminal side of lysine and arginine residues. Performic acid cleaves the disulfide bonds holding together the three subunits of chymotrypsin. Correct Answerarrow_forward
 Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College
Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning
Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning





