
Organic Chemistry; Modified MasteringChemistry with Pearson eText -- ValuePack Access Card; Study Guide and Student Solutions Manual for Organic Chemistry, Books a la Carte Edition (7th Edition)
7th Edition
ISBN: 9780134240152
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9, Problem 61P
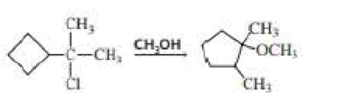
Propose a mechanism for the following reaction:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
1/2
-
51%
+ »
GAY
Organic Reactions Assignment
/26
Write the type of reaction that is occurring on the line provided then complete the reaction. Only include the
major products and any byproducts (e.g. H₂O) but no minor products. Please use either full structural
diagrams or the combination method shown in the lesson. Skeletal/line diagrams will not be accepted.
H3C
1.
2.
CH3
A
Acid
OH
Type of Reaction:
NH
Type of Reaction:
+ H₂O
Catalyst
+ HBr
3.
Type of Reaction:
H3C
4.
Type Reaction:
5. H3C
CH2 + H2O
OH
+
[0]
CH3
Type of Reaction:
6. OH
CH3
HO
CH3 +
Type of Reaction:
7.
Type of Reaction:
+ [H]
humbnai
Concentration Terms[1].pdf ox + New
Home
Edit
Sign in
Comment
Convert
Page
Fill & Sign
Protect
Tools
Batch
+WPS A
Free Trial
Share
Inter Concreting Concentration forms.
Hydrogen peroxide is
a powerful oxidizing agent
wed in concentrated solution in rocket fuels and
in dilute solution as a
hair bleach. An aqueous
sulation of H2O2 is 30% by mass and has
density of #liligime calculat the
Ⓒmolality
⑥mole fraction of
molarity.
20
9.
B. A sample of Commercial Concentrated hydrochloric
ET
If a reaction occurs, what would be the major products? Please include a detailed explanation as well as a drawing showing how the reaction occurs and what the final product is.
Chapter 9 Solutions
Organic Chemistry; Modified MasteringChemistry with Pearson eText -- ValuePack Access Card; Study Guide and Student Solutions Manual for Organic Chemistry, Books a la Carte Edition (7th Edition)
Ch. 9.1 - Prob. 3PCh. 9.1 - Does increasing the energy barrier for an SN2...Ch. 9.1 - Rank the following alkyl bromides from most...Ch. 9.2 - Prob. 8PCh. 9.2 - Prob. 9PCh. 9.2 - Prob. 10PCh. 9.2 - Prob. 11PCh. 9.2 - Which substitution reaction lakes place more...Ch. 9.2 - Prob. 14PCh. 9.2 - Prob. 16P
Ch. 9.3 - Prob. 17PCh. 9.4 - Prob. 18PCh. 9.5 - Prob. 19PCh. 9.5 - Prob. 20PCh. 9.5 - Prob. 21PCh. 9.5 - Prob. 22PCh. 9.6 - Prob. 23PCh. 9.6 - Prob. 24PCh. 9.6 - Which of the following reactions take place more...Ch. 9.7 - Prob. 26PCh. 9.7 - Prob. 27PCh. 9.7 - Prob. 28PCh. 9.7 - Prob. 30PCh. 9.7 - Under which of the following reaction conditions...Ch. 9.8 - After a proton is removed from the OH group, which...Ch. 9.8 - Prob. 33PCh. 9.9 - Prob. 34PCh. 9 - Prob. 1PCh. 9 - Methoxychlor is an insecticide that was intended...Ch. 9 - Prob. 35PCh. 9 - Prob. 36PCh. 9 - Prob. 37PCh. 9 - Prob. 38PCh. 9 - Prob. 39PCh. 9 - Prob. 40PCh. 9 - Starting with cyclohexene, how can the following...Ch. 9 - Prob. 42PCh. 9 - The pKa of acetic acid in water is 4.76. What...Ch. 9 - Prob. 44PCh. 9 - Prob. 45PCh. 9 - Prob. 46PCh. 9 - Prob. 47PCh. 9 - Prob. 48PCh. 9 - Prob. 49PCh. 9 - Prob. 50PCh. 9 - Prob. 51PCh. 9 - tert-Butyl chloride undergoes solvolysis in both...Ch. 9 - Prob. 53PCh. 9 - Prob. 54PCh. 9 - In which solventethanol or diethyl etherwould the...Ch. 9 - Prob. 56PCh. 9 - Two bromoethers are obtained from the reaction of...Ch. 9 - Prob. 58PCh. 9 - Prob. 59PCh. 9 - Prob. 60PCh. 9 - Propose a mechanism for the following reaction:Ch. 9 - Prob. 62PCh. 9 - Prob. 63PCh. 9 - Prob. 64PCh. 9 - Prob. 65PCh. 9 - When equivalent amounts of methyl bromide nod...Ch. 9 - Prob. 67PCh. 9 - The reaction of chloromethane with hydroxide ion...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Would the following organic synthesis occur in one step? Add any missing products, required catalysts, inorganic reagents, and other important conditions. Please include a detailed explanation and drawings showing how the reaction may occur in one step.arrow_forward(a) Sketch the 'H NMR of the following chemical including the approximate chemical shifts, the multiplicity (splitting) of all signals and the integration (b) How many signals would you expect in the 13C NMR? CH3arrow_forwardDraw the Show the major and minor product(s) for the following reaction mechanisms for both reactions and show all resonance structures for any Explain why the major product is favoured? intermediates H-Brarrow_forward
- 3. Draw ALL THE POSSBILE PRODUCTS AND THE MECHANISMS WITH ALL RESONANCE STRUCTURES. Explain using the resonance structures why the major product(s) are formed over the minor product(s). H₂SO4, HONO CHarrow_forward7. Provide the product(s), starting material(s) and/or condition(s) required for the No mechanisms required. below reaction HO + H-I CI FO Br2, FeBr3 O I-Oarrow_forward6. Design the most efficient synthesis of the following product starting from phenot Provide the reaction conditions for each step (more than one step is required) and explain the selectivity of each reaction. NO MECHANISMS ARE REQUIRED. OH step(s) CIarrow_forward
- What is the skeletal structure of the product of the following organic reaction?arrow_forwardIf a reaction occurs, what would be the major products? Please include a detailed explanation as well as a drawing showing how the reaction occurs and what the final product is.arrow_forwardWhat is the major organic product of the following nucleophilic acyl substitution reaction of an acid chloride below?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License