
Concept explainers
Interpretation:
The bond order and magnetic properties of the given molecules are to be determined.
Concept introduction:
Two atomic orbitals combine to form a bonding molecular orbital and an antibonding molecular orbital. Orbitals that lie on internuclear axis combine to form
(sigma) molecular orbitals, and orbitals parallel to each other combine to form
molecular orbitals.
The molecular orbitals formed by the combination of
orbitals are a bonding molecular orbital, designated by
orbital forms corresponding molecular orbitals.
The molecular orbitals formed by the combination of
orbitals area bonding molecular orbital, designated by
The molecular orbitals formed by combining
orbitals are bonding molecular orbitals, designated by
and
and
Electrons are filled in the molecular orbitals in increasing order of energy.
Bond order is determined by subtracting the number of electrons in antibonding orbitals from the number of electrons in bonding orbitals, and dividing by two.
Higher the bond order, more stable is the molecule.
A diamagnetic substance contains paired electrons whereas a paramagnetic substance contains unpaired electrons.
Answer to Problem 57QP
Solution:
Bond order of
is diamagnetic and
are paramagnetic.
Explanation of Solution
The electronic configuration of an oxygen atom is
is as follows:
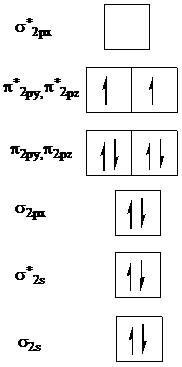
Now, remove one electron from
to get the molecular orbital diagram for
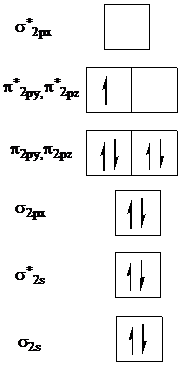
Add one electron to
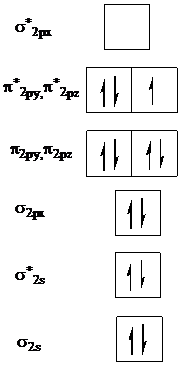
Add two electrons to
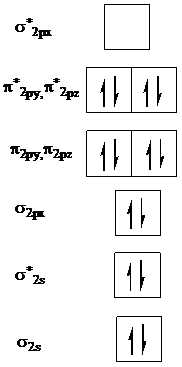
In
In
is calculated as follows:
In
In
is calculated as follows:
A molecule of
contains two unpaired electrons,
contains one unpaired electron,
contains one unpaired electron, and
contains no unpaired electron. Thus,
is diamagnetic and
are paramagnetic.
Want to see more full solutions like this?
Chapter 9 Solutions
BURDGE CHEMISTRY VALUE ED (LL)
- Polymers may be composed of thousands of monomers. Draw three repeat units (trimer) of the polymer formed in this reaction. Assume there are hydrogen atoms there are hydrogen atoms on the two ends of the trimer. Ignore inorganic byproducts please.arrow_forwardi need help with the folarrow_forwardPLEASE HELP NOW! URGENT!arrow_forward
- a. Determine whether each of the Followery Molecules is in the R- On the y- Configuration 1-01"/ 1-6-4 Br 4 I el Br b. Draw The Fisher projection For all the Meso compounds that can exist FOR The Following molenlearrow_forward1- Refer to the monosaccharides below to answer each of the following question(s): CH₂OH CHO CH₂OH CH₂OH 0 H- OH 0 0 HO- H H- -OH HO H HO H H OH HO- H CH₂OH H. OH HO H HO- H CH₂OH CH₂OH CH3 a. Sorbose b. Rhamnose c. Erythrulose d. Xylulose Classify each sugar by type; for example, glucose is an aldohexose. a. Xylulose is .. b. Erythrulose is . c. Sorbose is .. d. Rhamnose is .. 2- Consider the reaction below to answer the following question(s). CHO H OH CH₂OH CH₂OH HO- H HO HO + H. -OH HO OH HO. H OH OH H -OH H OH CH₂OH Q Z a. Refer to Exhibit 25-11. Place a triangle around the anomeric carbon in compound Q. Compound Z is: b. 1. the D-anomer. 2. the a-anomer. 3. the ẞ-anomer. 4. the L-anomer. c. Which anomer is the LEAST stable? d. Q and Z are cyclic examples of: a. acetals b. hemiacetals c. alditols d. hemialditolsarrow_forwardi need help identifying the four carbon oxygen bonds in the following:arrow_forward
- Imagine each of the molecules shown below was found in an aqueous solution. Can you tell whether the solution is acidic, basic, or neutral? molecule HO H3N + The solution is... X O acidic OH O basic H3N-CH-C-O O neutral ○ (unknown) O acidic ○ basic CH2 CH 3-S-CH2 O neutral ○ (unknown) H3N O OH O acidic O basic Oneutral O (unknown) 0 H3N-CH-C-O CH3 CH CH3 O acidic O basic O neutral ○ (unknown) ? olo Ar BHarrow_forwardno Ai walkthroughs need other product (product in picture is wrong dont submit the same thing)arrow_forwardHow to solve this!arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





