
Interpretation:
A balanced equation for the given hypothetical reaction is to be written, and the oxidation and hybridization state of sulfur in
required and the mass of
produced is to be calculated.
Concept introduction:
The oxidation state of an element is zero. The sum of the oxidation states of all the elements is equal to zero in a molecule of a compound, and in case of an ion, it is equal to the charge on the ion. Oxygen has a fixed oxidation state of
in its compounds.
To find hybridisation of an atom in a molecule, at first draw the Lewis structure of the molecule. Find the number of electrons domains around the atom. This gives the number of hybrid orbitals required for bonding. The number of hybrid orbitals is equal to the number of atomic orbitals that hybridise. Thus, one
and one
orbital hybridize to form two
and two
orbitals hybridize to form three
hybrid orbitals, and one
and three
orbitals hybridize to form four
hybrid orbitals.
The conversion factor is a fraction that is used to convert one unit to another. Use of more than one factor to find a solution is called dimensional analysis.
Answer to Problem 115AP
Solution: The balanced equation for the reaction is as follows:
The oxidation state of sulfur in elemental sulfur
is
is
is
is
is
and mass of
produced is
Explanation of Solution
The hypothetical reaction between elemental sulfur and sulfur trioxide is as follows:
The balanced equation for this reaction is as follows:
In
The oxidation state of oxygen is
Let
is the oxidation state of sulfur.
Then
is calculated as follows:
The oxidation state of sulfur in
is
In
Let
is the oxidation state of sulfur and the oxidation state of oxygen is
is calculated as follows:
The oxidation state of sulfur in
is
The Lewis structure of
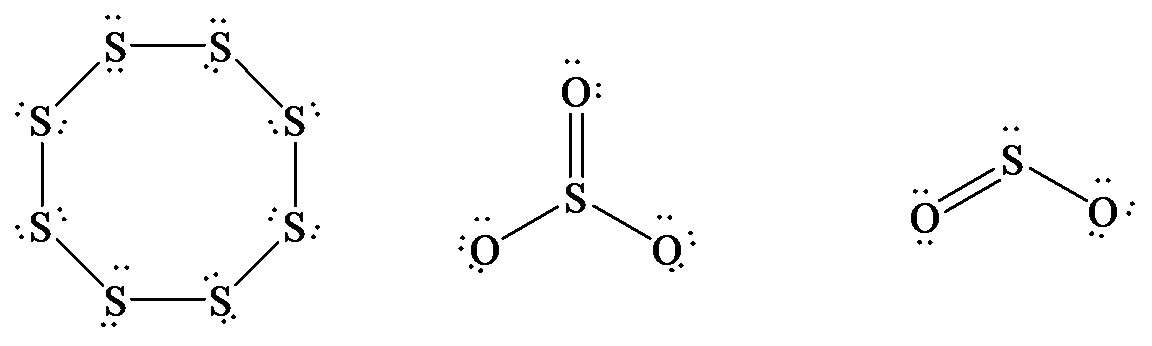
In
hybrid orbitals.
In
hybrid orbitals.
In
hybrid orbitals.
Consider the balanced equation:
One mole of
combines with
moles of
moles of
is
is given as
Convert the mass of
to moles as follows:
One mole of
combines with
moles of
Thus, for
needed is calculated as follows:
Molar mass of
is
Thus, the amount of
One mole of
produces
moles of
Thus, for
produced is calculated as follows:
Molar mass of
is
Thus, the amount of
Hence, mass of
required is
produced is
The balanced equation for the reaction between elemental sulfur and sulfur trioxide to produce sulfur dioxide is written; the oxidation and hybridization state of sulfur in
required and the mass of
produced by
of elemental sulfur are calculated.
Want to see more full solutions like this?
Chapter 9 Solutions
BURDGE CHEMISTRY VALUE ED (LL)
- i need help identifying the four carbon oxygen bonds in the following:arrow_forwardImagine each of the molecules shown below was found in an aqueous solution. Can you tell whether the solution is acidic, basic, or neutral? molecule HO H3N + The solution is... X O acidic OH O basic H3N-CH-C-O O neutral ○ (unknown) O acidic ○ basic CH2 CH 3-S-CH2 O neutral ○ (unknown) H3N O OH O acidic O basic Oneutral O (unknown) 0 H3N-CH-C-O CH3 CH CH3 O acidic O basic O neutral ○ (unknown) ? olo Ar BHarrow_forwardno Ai walkthroughs need other product (product in picture is wrong dont submit the same thing)arrow_forward
- I have a 2 mil plastic film that degrades after 22 days at 88C and at 61C takes 153 days. What is the failure at 47C in days.arrow_forwardIf a 5 film plastic film degraded in 30 days at 35C and the same film degraded in 10 days at 55 C and 2 days at 65C what would the predicted life time be at 22C for the same film?arrow_forwardno Ai walkthroughsarrow_forward
- I have a aqueous solution (175 ml) of iridium trichloride containing 8,750 ppm Iridium by ICP OES analysis. What is the percent concentration of Iridium trichloride in aquous solution and provide the concentration in moles per liter, percentage by weight.arrow_forwardno Ai walkthroughsarrow_forwardno Ai walkthroughsarrow_forward
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





