
Concept explainers
Interpretation:
The hybrid orbitals of carbon atoms in the given molecules are to be determined.
Concept introduction:
Hybridization is the combining of atomic orbitals to form hybrid orbitals.
To determine hybridization of an atom, first draw the Lewis structure of the molecule.
Find the number of electron domains around an atom so as to get the number of hybrid orbitals used by the atom for bonding.
When atomic orbitals combine, they form equal number of hybrid orbitals.
The s orbital combines with one, two, or three p orbitals to form
Answer to Problem 36QP
Solution:
a)
b)
c)
d)
e)
a)
Explanation of Solution
The Lewis structure of
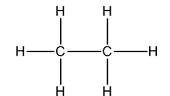
In this, the two carbon atoms are bonded to each other and the three hydrogen atoms by single bonds. Thus, there are four electron domains around each carbon atom. Four electron domainsdepict thepresence of four hybrid orbitals. Thus, in both carbon atoms
b)
The Lewis structure of
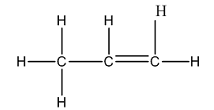
The carbon on the left is bonded to three hydrogen atoms by single bonds and to a carbon atom by a single bond. Thus, there are four electron domains around each carbon atom. Four electron domainsdepict thepresence of four hybrid orbitals. Thus, in this carbon
The central carbon is bonded to one carbon atom by a single bond, to another by double bond and to a hydrogen by a single bond. Thus, there are three electron domains around this carbon atom. Three electron domainsdepict thepresence of three hybrid orbitals. Thus, hybridization of the central carbon is
The carbon on the right is bonded to two hydrogen atoms by a single bond and to a carbon by a double bond. Thus, there are three electron domains around this carbon atom. Three electron domainsdepict thepresence of three hybrid orbitals. Thus, hybridization of the carbon on the right is
Thus, the hybridizations of the carbon atoms, from left to right in the molecule, are
c)
The Lewis structure of
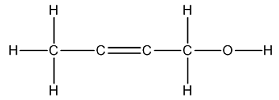
The carbon on left is bonded to three hydrogen atoms by single bonds and to a carbon atom by single bond. Thus, there are four electron domains around each carbon atom. Four electron domainsdepict thepresence of four hybrid orbitals. Thus, in this carbon,
The two carbon atoms in the center are bonded to each other by double bonds and to a carbon by a single bond. Thus, there are two electron domains around each carbon atom. Two electron domains depict the presence of two hybrid orbitals. Thus, in these two carbons, sp hybrid orbitals are present.
The carbon on the right is bonded to two hydrogen atoms by single bonds, to a carbon atom by a single bond and to an oxygen by a single bond. Thus, there are four electron domains around this carbon atom. Four electron domainsdepict the presence of four hybrid orbitals. Thus, in this carbon,
Thus, the hybridizations of the carbon atoms from left to right in the molecule are
d)
The Lewis structure of
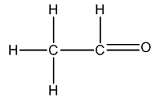
The carbon on the left is bonded to three hydrogen atoms by single bonds and to a carbon atom by a single bond. Thus, there are four electron domains around each carbon atom. Four electron domainsdepict the presence of four hybrid orbitals. Thus, in this carbon
The carbon on the right is bonded to a hydrogen atom by a single bond and to an oxygen atom by a double bond. Thus, there are three electron domains around this carbon atom. Three electron domainsdepict the presence of three hybrid orbitals. Thus, the hybridization of the carbon atom on the right is
Thus, the hybridizations of carbon atoms from left to right in the molecule are
e)
The Lewis structure of
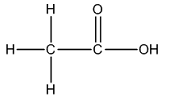
The carbon on left is bonded to three hydrogen atoms by single bonds and to carbon atom by single bond. Thus, there are four electron domains around each carbon atom. Four electron domainsdepict the presence of four hybrid orbitals. Thus, in this carbon
The carbon on the right is bonded to an oxygen atom by a double bond and to another oxygen atom by a single bond. Thus, there are three electron domains around this carbon atom. Three electron domainsdepict the presence of three hybrid orbitals. Thus, the hybridization of the carbonatomon the right is
Thus, the hybridizations of carbon atoms from left to right in the molecule are
Want to see more full solutions like this?
Chapter 9 Solutions
EBK CHEMISTRY
- Indicate the product(s) B and C that are formed in the reaction: HN' OCH HC1 B + mayoritario C minoritario OCH3arrow_forwardIndicate the product(s) that are formed in the reaction: NH-NH, OCH3 -H₂O OCH3arrow_forward21.38 Arrange the molecules in each set in order of increasing acidity (from least acidic to most acidic). OH OH SH NH2 8 NH3 OH (b) OH OH OH (c) & & & CH3 NO2 21.39 Explain the trends in the acidity of phenol and the monofluoro derivatives of phenol. OH OH OH OH PK 10.0 PK 8.81 PK 9.28 PK 9.81arrow_forward
- identify which spectrum is for acetaminophen and which is for phenacetinarrow_forwardThe Concept of Aromaticity 21.15 State the number of 2p orbital electrons in each molecule or ion. (a) (b) (e) (f) (c) (d) (h) (i) DA (k) 21.16 Which of the molecules and ions given in Problem 21.15 are aromatic according to the Hückel criteria? Which, if planar, would be antiaromatic? 21.17 Which of the following structures are considered aromatic according to the Hückel criteria? ---0-0 (a) (b) (c) (d) (e) (h) H -H .8.0- 21.18 Which of the molecules and ions from Problem 21.17 have electrons donated by a heteroatom?arrow_forward1. Show the steps necessary to make 2-methyl-4-nonene using a Wittig reaction. Start with triphenylphosphine and an alkyl halide. After that you may use any other organic or inorganic reagents. 2. Write in the product of this reaction: CH3 CH₂ (C6H5)₂CuLi H₂O+arrow_forward
- 3. Name this compound properly, including stereochemistry. H₂C H3C CH3 OH 4. Show the step(s) necessary to transform the compound on the left into the acid on the right. Bri CH2 5. Write in the product of this LiAlH4 Br H₂C OHarrow_forwardWhat are the major products of the following reaction? Please provide a detailed explanation and a drawing to show how the reaction proceeds.arrow_forwardWhat are the major products of the following enolate alkylation reaction? Please include a detailed explanation as well as a drawing as to how the reaction proceeds.arrow_forward
- A block of zinc has an initial temperature of 94.2 degrees celcius and is immererd in 105 g of water at 21.90 degrees celcius. At thermal equilibrium, the final temperature is 25.20 degrees celcius. What is the mass of the zinc block? Cs(Zn) = 0.390 J/gxdegrees celcius Cs(H2O) = 4.18 J/gx degrees celcusarrow_forwardPotential Energy (kJ) 1. Consider these three reactions as the elementary steps in the mechanism for a chemical reaction. AH = -950 kJ AH = 575 kJ (i) Cl₂ (g) + Pt (s) 2C1 (g) + Pt (s) Ea = 1550 kJ (ii) Cl (g)+ CO (g) + Pt (s) → CICO (g) + Pt (s) (iii) Cl (g) + CICO (g) → Cl₂CO (g) Ea = 2240 kJ Ea = 2350 kJ AH = -825 kJ 2600 2400 2200 2000 1800 1600 1400 1200 1000 a. Draw the potential energy diagram for the reaction. Label the data points for clarity. The potential energy of the reactants is 600 kJ 800 600 400 200 0 -200- -400 -600- -800- Reaction Progressarrow_forwardCan u help me figure out the reaction mechanisms for these, idk where to even startarrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

