
Chemistry with Access Code, Hybrid Edition
9th Edition
ISBN: 9781285188492
Author: Steven S. Zumdahl
Publisher: CENGAGE L
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9, Problem 32E
The allene molecule has the following Lewis structure:
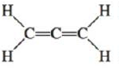
Must all hydrogen atoms lie the same plane? If not, what is their spatial relationship? Explain.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Using reaction free energy to predict equilibrium composition
Consider the following equilibrium:
2NH3 (g) = N2 (g) +3H₂
—N2 (g) AGº = 34. kJ
Now suppose a reaction vessel is filled with 4.19 atm of ammonia (NH3) and 9.94 atm of nitrogen (N2) at 378. °C. Answer the following questions about this
system:
rise
Under these conditions, will the pressure of NH 3 tend to rise or fall?
☐ x10
fall
Х
Is it possible to reverse this tendency by adding H₂?
In other words, if you said the pressure of NH 3 will tend to rise, can that
be changed to a tendency to fall by adding H₂? Similarly, if you said the
pressure of NH3 will tend to fall, can that be changed to a tendency to
rise by adding H₂?
If you said the tendency can be reversed in the second question, calculate
the minimum pressure of H₂ needed to reverse it.
Round your answer to 2 significant digits.
yes
no
atm
00.
18
Ar
무ㅎ
?
Identifying the major species in weak acid or weak base equilibria
The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at
equilibrium. You can leave out water itself.
Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the
formulas of the species that will act as neither acids nor bases in the 'other' row.
You will find it useful to keep in mind that HF is a weak acid.
2.2 mol of NaOH is added to
1.0 L of a 1.4M HF
solution.
acids:
П
bases:
Х
other: ☐
ப
acids:
0.51 mol of KOH is added to
1.0 L of a solution that is
bases:
1.3M in both HF and NaF.
other: ☐
00.
18
Ar
Using reaction free energy to predict equilibrium composition
Consider the following equilibrium:
N2O4 (g) 2NO2 (g)
AG⁰ = 5.4 kJ
Now suppose a reaction vessel is filled with 1.68 atm of dinitrogen tetroxide (N204) at 148. °C. Answer the following questions about this system:
rise
Under these conditions, will the pressure of N2O4 tend to rise or fall?
x10
fall
Is it possible to reverse this tendency by adding NO2?
In other words, if you said the pressure of N2O4 will tend to rise, can that
be changed to a tendency to fall by adding NO2? Similarly, if you said the
pressure of N2O4 will tend to fall, can that be changed to a tendency to
rise by adding NO2?
If you said the tendency can be reversed in the second question, calculate
the minimum pressure of NO 2 needed to reverse it.
Round your answer to 2 significant digits.
yes
no
0.42 atm
☑
5
0/5
?
مله
Ar
Chapter 9 Solutions
Chemistry with Access Code, Hybrid Edition
Ch. 9 - Why do we hybtidize atomic orbitals to explain the...Ch. 9 - What hybridization is required for central atoms...Ch. 9 - Describe the bonding in H2S, CH4, H2CO and HCN...Ch. 9 - What hybridization is required for central atoms...Ch. 9 - Electrons in bonding molecular orbitals are most...Ch. 9 - What are molecular orbitals? How do they compare...Ch. 9 - Explain the difference between the and MOs for...Ch. 9 - Compare Figs. 4-47 and 4-49. Why are they...Ch. 9 - Which of the following would you expect to be more...Ch. 9 - Draw the Lewis structure for HCN. Indicate the...
Ch. 9 - Which is the more correct statement: The methane...Ch. 9 - Compare and contrast the MO model with the local...Ch. 9 - What are the relationships among bond order, bond...Ch. 9 - In the hybrid orbital model, compare and contrast ...Ch. 9 - In the molecular orbital mode l, compare and...Ch. 9 - Why are d orbitals sometimes used to form hybrid...Ch. 9 - The atoms in a single bond can rotate about the...Ch. 9 - Compare and contrast bonding molecular orbitals...Ch. 9 - What modification to the molecular orbital model...Ch. 9 - Why does the molecular orbital model do a better...Ch. 9 - The three NO bonds in NO3 are all equivalent in...Ch. 9 - Use the localized electron model to describe the...Ch. 9 - Use the localized electron model to describe the...Ch. 9 - Use the localized electron model to describe the...Ch. 9 - Use the localized electron model to describe the...Ch. 9 - The space-filling models of ethane and ethanol are...Ch. 9 - The space-filling models of hydrogen cyanide and...Ch. 9 - Give the expected hybridization of the central...Ch. 9 - Give the expected hybridization of the central...Ch. 9 - Give the expected hybridization of the central...Ch. 9 - Give the expected hybridization of the central...Ch. 9 - For each of the following molecules, write the...Ch. 9 - For each of the following molecules or ions that...Ch. 9 - Prob. 31ECh. 9 - The allene molecule has the following Lewis...Ch. 9 - Indigo is the dye used in coloring blue jeans. The...Ch. 9 - Urea, a compound formed in the liver, is one of...Ch. 9 - Biacetyl and acetoin are added to margarine to...Ch. 9 - Many important compounds in the chemical industry...Ch. 9 - Two molecules used in the polymer industry are...Ch. 9 - Hot and spicy foods contain molecules that...Ch. 9 - One of the first drugs to be approved for use in...Ch. 9 - The antibiotic thiarubin-A was discovered by...Ch. 9 - Consider the following molecular orbitals formed...Ch. 9 - Sketch the molecular orbital and label its type (...Ch. 9 - Which of the following are predicted by the...Ch. 9 - Which of the following are predicted by the...Ch. 9 - Using the molecular orbital model, write electron...Ch. 9 - Consider the following electron configuration:...Ch. 9 - Using molecular orbital theory, explain why the...Ch. 9 - Using the molecular orbital model to describe the...Ch. 9 - The transport of O2 in the blood is carried out by...Ch. 9 - A Lewis structure obeying the octet rule can be...Ch. 9 - Using the molecular orbital model, write electron...Ch. 9 - Using the molecular orbital model, write electron...Ch. 9 - In which of the following diatomic molecules would...Ch. 9 - In terms of the molecular orbital model, which...Ch. 9 - Show how two 2p atomic orbitals can combine to...Ch. 9 - Show how a hydrogen 1s atomic orbital and a...Ch. 9 - Use Figs. 4-54 and 4-55 to answer the following...Ch. 9 - Acetylene (C2H2) can be produced from the reaction...Ch. 9 - Describe the bonding in NO+, NO, and NO, using...Ch. 9 - Describe the bonding in the O3 molecule and the...Ch. 9 - Describe the bonding in the CO32 ion using the...Ch. 9 - Draw the Lewis structures, predict the molecular...Ch. 9 - FClO2 and F3ClO can both gain a fluoride ion to...Ch. 9 - Two structures can be drawn for cyanuric acid: a....Ch. 9 - Give the expected hybridization for the molecular...Ch. 9 - Vitamin B6 is an organic compound whose deficiency...Ch. 9 - Aspartame is an artificial sweetener marketed...Ch. 9 - Prob. 69AECh. 9 - The three most stable oxides of carbon are carbon...Ch. 9 - Complete the following resonance structures for...Ch. 9 - Prob. 73AECh. 9 - Describe the bonding in the first excited state of...Ch. 9 - Using an MO energy-level diagram, would you expect...Ch. 9 - Show how a dxz. atomic orbital and a pz, atomic...Ch. 9 - What type of molecular orbital would result from...Ch. 9 - Consider three molecules: A, B, and C. Molecule A...Ch. 9 - Draw the Lewis structures for TeCl4, ICl5, PCl5,...Ch. 9 - A variety of chlorine oxide fluorides and related...Ch. 9 - Pelargondin is the molecule responsible for the...Ch. 9 - Complete a Lewis structure for the compound shown...Ch. 9 - Which of the following statements concerning SO2...Ch. 9 - Consider the molecular orbital electron...Ch. 9 - Place the species B2+ , B2, and B2 in order of...Ch. 9 - Consider the following computer-generated model of...Ch. 9 - Cholesterol (C27liu;O) has the following...Ch. 9 - Cyanamide (H2NCN), an important industrial...Ch. 9 - A flask containing gaseous N2 is irradiated with...Ch. 9 - Prob. 92CPCh. 9 - Values of measured bond energies may vary greatly...Ch. 9 - Use the MO model to explain the bonding in BeH2....Ch. 9 - Prob. 95CPCh. 9 - Arrange the following from lowest to highest...Ch. 9 - Use the MO model to determine which of the...Ch. 9 - Given that the ionization energy of F2 is 290...Ch. 9 - Carbon monoxide (CO) forms bonds to a variety of...Ch. 9 - Prob. 100CPCh. 9 - As the bead engineer of your starship in charge of...Ch. 9 - Determine the molecular structure and...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Homework 13 (Ch17) Question 4 of 4 (1 point) | Question Attempt: 2 of 2 ✓ 1 ✓ 2 = 3 4 Time Remaining: 4:25:54 Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction free energy of the following chemical reaction: 2CH3OH (g)+302 (g) → 2CO2 (g) + 4H₂O (g) Round your answer to zero decimal places. ☐ kJ x10 ☐ Subm Check 2020 Hill LLC. All Rights Reserved. Terms of Use | Privacy Cearrow_forwardIdentifying the major species in weak acid or weak base equilibria Your answer is incorrect. • Row 2: Your answer is incorrect. • Row 3: Your answer is incorrect. • Row 6: Your answer is incorrect. 0/5 The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HF is a weak acid. acids: HF 0.1 mol of NaOH is added to 1.0 L of a 0.7M HF solution. bases: 0.13 mol of HCl is added to 1.0 L of a solution that is 1.0M in both HF and KF. Exponent other: F acids: HF bases: F other: K 1 0,0,... ? 000 18 Ararrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: 2NOCI (g) 2NO (g) + Cl2 (g) AGº =41. kJ Now suppose a reaction vessel is filled with 4.50 atm of nitrosyl chloride (NOCI) and 6.38 atm of chlorine (C12) at 212. °C. Answer the following questions about this system: ? rise Under these conditions, will the pressure of NOCI tend to rise or fall? x10 fall Is it possible to reverse this tendency by adding NO? In other words, if you said the pressure of NOCI will tend to rise, can that be changed to a tendency to fall by adding NO? Similarly, if you said the pressure of NOCI will tend to fall, can that be changed to a tendency to rise by adding NO? yes no If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO needed to reverse it. Round your answer to 2 significant digits. 0.035 atm ✓ G 00. 18 Ararrow_forward
- Highlight each glycosidic bond in the molecule below. Then answer the questions in the table under the drawing area. HO- HO- -0 OH OH HO NG HO- HO- OH OH OH OH NG OHarrow_forward€ + Suppose the molecule in the drawing area below were reacted with H₂ over a platinum catalyst. Edit the molecule to show what would happen to it. That is, turn it into the product of the reaction. Also, write the name of the product molecule under the drawing area. Name: ☐ H C=0 X H- OH HO- H HO- -H CH₂OH ×arrow_forwardDraw the Haworth projection of the disaccharide made by joining D-glucose and D-mannose with a ẞ(1-4) glycosidic bond. If the disaccharide has more than one anomer, you can draw any of them. Click and drag to start drawing a structure. Xarrow_forward
- Epoxides can be opened in aqueous acid or aqueous base to produce diols (molecules with two OH groups). In this question, you'll explore the mechanism of epoxide opening in aqueous acid. 2nd attempt Be sure to show all four bonds at stereocenters using hash and wedge lines. 0 0 Draw curved arrows to show how the epoxide reacts with hydronium ion. 100 +1: 1st attempt Feedback Be sure to show all four bonds at stereocenters using hash and wedge lines. See Periodic Table See Hint H A 5 F F Hr See Periodic Table See Hintarrow_forward03 Question (1 point) For the reaction below, draw both of the major organic products. Be sure to consider stereochemistry. > 1. CH₂CH₂MgBr 2. H₂O 3rd attempt Draw all four bonds at chiral centers. Draw all stereoisomers formed. Draw the structures here. e 130 AN H See Periodic Table See Hint P C Brarrow_forwardYou may wish to address the following issues in your response if they are pertinent to the reaction(s) you propose to employ:1) Chemoselectivity (why this functional group and not another?) 2) Regioselectivity (why here and not there?) 3) Stereoselectivity (why this stereoisomer?) 4) Changes in oxidation state. Please make it in detail and draw it out too in what step what happens. Thank you for helping me!arrow_forward
- 1) Chemoselectivity (why this functional group and not another?) 2) Regioselectivity (why here and not there?) 3) Stereoselectivity (why this stereoisomer?) 4) Changes in oxidation state. Everything in detail and draw out and write it.arrow_forwardCalculating the pH at equivalence of a titration 3/5 Izabella A chemist titrates 120.0 mL of a 0.7191M dimethylamine ((CH3)2NH) solution with 0.5501 M HBr solution at 25 °C. Calculate the pH at equivalence. The pk of dimethylamine is 3.27. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of HBr solution added. pH = ☐ ✓ 18 Ar Boarrow_forwardAlcohols can be synthesized using an acid-catalyzed hydration of an alkene. An alkene is combined with aqueous acid (e.. sulfuric acid in water). The reaction mechanism typically involves a carbocation intermediate. > 3rd attempt 3343 10 8 Draw arrows to show the reaction between the alkene and hydronium ion. that 2nd attempt Feedback 1st attempt تعمال Ju See Periodic Table See Hint F D Ju See Periodic Table See Hintarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
NEET Chemistry | Group 14 Carbon Family | Theory & Problem Solving | In English | Misostudy; Author: Misostudy;https://www.youtube.com/watch?v=enOGIrcHh54;License: Standard YouTube License, CC-BY