
General Chemistry: Atoms First
2nd Edition
ISBN: 9780321809261
Author: John E. McMurry, Robert C. Fay
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 8.4, Problem 8.4CP
The following reaction has ΔE = −186 kJ/mol.
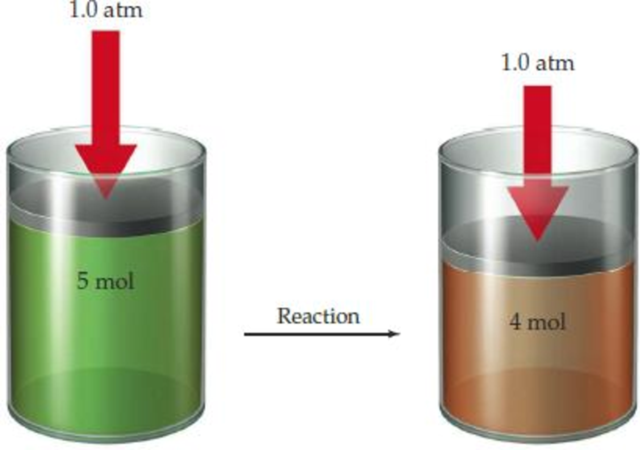
- (a) Is the sign of PΔV positive or negative? Explain.
- (b) What is the sign and approximate magnitude of ΔH? Explain.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
5.
6.
0/5
alekscgi/x/sl.exe/1o_u-IgNglkr7j8P3jH-IQs_pBaHhvlTCeeBZbufuBYTi0Hz7m7D3ZcSLEFovsXaorzoFtUs | AbtAURtkqzol 1HRAS286,
O States of Matter
Sketching a described thermodynamic change on a phase diagram
The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the
temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes.
3
pressure (atm)
+
0-
0
5+
200
temperature (K)
400
Explanation
Check
X
0+
F3
F4
F5
F6
F7
S
2025 McGraw Hill LLC All Rights Reserved. Terms of Use Privacy Center
Accessibility
Q Search
LUCR
+
F8
F9
F10
F11
F12
*
%
&
(
5
6
7
8
9
Y'S
Dele
Insert
PrtSc
+
Backs
Chapter 8 Solutions
General Chemistry: Atoms First
Ch. 8.2 - Which of the following are state functions, and...Ch. 8.3 - Calculate the work in kilojoules done during a...Ch. 8.3 - How much work is done in kilojoules, and in which...Ch. 8.4 - The following reaction has E = 186 kJ/mol. (a) Is...Ch. 8.5 - Assuming that Coca Cola has the same specific heat...Ch. 8.5 - What is the specific heat of lead if it takes 97.2...Ch. 8.5 - When 25.0 mL of 1.0 M H2SO4 is added to 50.0 mL of...Ch. 8.6 - The reaction between hydrogen and oxygen to yield...Ch. 8.6 - The explosion of 2.00 mol of solid trinitrotoluene...Ch. 8.7 - How much heat in kilojoules is evolved or absorbed...
Ch. 8.7 - Nitromethane (CH3NO2), sometimes used as a fuel in...Ch. 8.8 - The industrial degreasing solvent methylene...Ch. 8.8 - The reaction of A with B to give D proceeds in two...Ch. 8.8 - Draw a Hesss law diagram similar to that in...Ch. 8.9 - Use the information in Table 8.2 to calculate H in...Ch. 8.9 - Use the information in Table 8.2 to calculate H in...Ch. 8.10 - Use the data in Table 8.3 to calculate an...Ch. 8.10 - Use the data in Table 8.3 to calculate an...Ch. 8.11 - Liquid butane (C4H10), the fuel used in many...Ch. 8.12 - Ethane, C2H6, can be prepared by the reaction of...Ch. 8.12 - Is the reaction represented in the following...Ch. 8.12 - Which of the following reactions are spontaneous...Ch. 8.12 - Is the Haber process for the industrial synthesis...Ch. 8.12 - The following reaction is exothermic: (a) Write a...Ch. 8.12 - Write balanced equations for the combustion...Ch. 8.12 - Biodiesel has a more favorable (more negative)...Ch. 8 - The following reaction is exothermic: (a) Write a...Ch. 8 - Imagine a reaction that results in a change in...Ch. 8 - Redraw the following diagram to represent the...Ch. 8 - Prob. 8.30CPCh. 8 - Prob. 8.31CPCh. 8 - A reaction is carried out in a cylinder fitted...Ch. 8 - The following drawing portrays a reaction of the...Ch. 8 - Prob. 8.34CPCh. 8 - The following reaction of A3 molecules is...Ch. 8 - Prob. 8.36SPCh. 8 - What is internal energy?Ch. 8 - Prob. 8.38SPCh. 8 - Assume that the kinetic energy of a 1400 kg car...Ch. 8 - Prob. 8.40SPCh. 8 - The addition of H2 to CC double bonds is an...Ch. 8 - Prob. 8.42SPCh. 8 - Prob. 8.43SPCh. 8 - Prob. 8.44SPCh. 8 - Prob. 8.45SPCh. 8 - Prob. 8.46SPCh. 8 - Does a measurement carried out in a bomb...Ch. 8 - Prob. 8.48SPCh. 8 - Prob. 8.49SPCh. 8 - Prob. 8.50SPCh. 8 - When 0.187 g of benzene, C6H6, is burned in a bomb...Ch. 8 - When a solution containing 8.00 g of NaOH in 50.0...Ch. 8 - Prob. 8.53SPCh. 8 - Prob. 8.54SPCh. 8 - Prob. 8.55SPCh. 8 - Prob. 8.56SPCh. 8 - Prob. 8.57SPCh. 8 - Prob. 8.58SPCh. 8 - Prob. 8.59SPCh. 8 - Prob. 8.60SPCh. 8 - Prob. 8.61SPCh. 8 - Used in welding metals, the reaction of acetylene...Ch. 8 - Prob. 8.63SPCh. 8 - The familiar ether used as an anesthetic agent is...Ch. 8 - How much energy in kilojoules is required to...Ch. 8 - Prob. 8.66SPCh. 8 - Prob. 8.67SPCh. 8 - Prob. 8.68SPCh. 8 - Prob. 8.69SPCh. 8 - Prob. 8.70SPCh. 8 - Prob. 8.71SPCh. 8 - Prob. 8.72SPCh. 8 - Prob. 8.73SPCh. 8 - Prob. 8.74SPCh. 8 - Prob. 8.75SPCh. 8 - Prob. 8.76SPCh. 8 - Prob. 8.77SPCh. 8 - Prob. 8.78SPCh. 8 - Prob. 8.79SPCh. 8 - Prob. 8.80SPCh. 8 - Prob. 8.81SPCh. 8 - Styrene (C8H8), the precursor of polystyrene...Ch. 8 - Prob. 8.83SPCh. 8 - Prob. 8.84SPCh. 8 - Prob. 8.85SPCh. 8 - Prob. 8.86SPCh. 8 - Prob. 8.87SPCh. 8 - Use the bond dissociation energies in Table 8.3 on...Ch. 8 - Use the bond dissociation energies in Table 8.3 to...Ch. 8 - Prob. 8.90SPCh. 8 - Prob. 8.91SPCh. 8 - Prob. 8.92SPCh. 8 - Prob. 8.93SPCh. 8 - Prob. 8.94SPCh. 8 - Prob. 8.95SPCh. 8 - Prob. 8.96SPCh. 8 - Prob. 8.97SPCh. 8 - Prob. 8.98SPCh. 8 - Prob. 8.99SPCh. 8 - Prob. 8.100SPCh. 8 - Prob. 8.101SPCh. 8 - Prob. 8.102SPCh. 8 - Tell whether reactions with the following values...Ch. 8 - Prob. 8.104SPCh. 8 - Prob. 8.105SPCh. 8 - Prob. 8.106SPCh. 8 - Prob. 8.107SPCh. 8 - Prob. 8.108SPCh. 8 - Prob. 8.109SPCh. 8 - When 1.50 g of magnesium metal is allowed to react...Ch. 8 - Use the data in Appendix B to find standard...Ch. 8 - Prob. 8.112CHPCh. 8 - The boiling point of a substance is defined as the...Ch. 8 - What is the melting point of benzene in kelvin if...Ch. 8 - Metallic mercury is obtained by heating the...Ch. 8 - Prob. 8.116CHPCh. 8 - Methanol (CH3OH) is made industrially in two steps...Ch. 8 - Isooctane, C8H18, is the component of gasoline...Ch. 8 - We said in Section 8.1 that the potential energy...Ch. 8 - For a process to be spontaneous, the total entropy...Ch. 8 - Set up a Hesss law cycle, and use the following...Ch. 8 - Prob. 8.122CHPCh. 8 - Prob. 8.123CHPCh. 8 - Prob. 8.124CHPCh. 8 - Citric acid has three dissociable hydrogens. When...Ch. 8 - Prob. 8.126CHPCh. 8 - Imagine that you dissolve 10.0 g of a mixture of...Ch. 8 - Prob. 8.128CHPCh. 8 - Prob. 8.129MPCh. 8 - Phosgene, COCl2(g), is a toxic gas used as an...Ch. 8 - Prob. 8.131MPCh. 8 - (a) Write a balanced equation for the reaction of...Ch. 8 - Prob. 8.133MPCh. 8 - Reaction of gaseous fluorine with compound X...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 5.arrow_forward9arrow_forwardalekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IQs_pBanHhvlTCeeBZbufu BYTI0Hz7m7D3ZS18w-nDB10538ZsAtmorZoFusYj2Xu9b78gZo- O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 3- 200 temperature (K) Explanation Chick Q Sowncharrow_forward
- 0+ aleksog/x/lsl.exe/1ou-lgNgkr7j8P3H-IQs pBaHhviTCeeBZbufuBYTOHz7m7D3ZStEPTBSB3u9bsp3Da pl19qomOXLhvWbH9wmXW5zm O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 Gab The temperature on a sample of pure X held at 0.75 atm and -229. °C is increased until the sample sublimes. The temperature is then held constant and the pressure is decreased by 0.50 atm. On the phase diagram below draw a path that shows this set of changes. F3 pressure (atm) 0- 0 200 Explanation temperature (K) Check F4 F5 ☀+ Q Search Chill Will an 9 ENG F6 F7 F8 F9 8 Delete F10 F11 F12 Insert PrtSc 114 d Ararrow_forwardx + LEKS: Using a phase diagram a X n/alekscgi/x/lsl.exe/10_u-IgNsikr7j8P3jH-IQs_pBan HhvlTCeeBZbufu BYTI0Hz7m7D3ZcHYUt80XL-5alyVpw ○ States of Matter Using a phase diagram to find a phase transition temperature or pressure Use the phase diagram of Substance X below to find the melting point of X when the pressure above the solid is 1.1 atm. pressure (atm) 16 08- solid liquid- 0 200 400 gas 600 temperature (K) Note: your answer must be within 25 °C of the exact answer to be graded correct. × 5arrow_forwardS: Using a phase diagram leksogi/x/sl.exe/1ou-IgNs kr 7j8P3jH-IQs_pBan HhvTCeeBZbufuBYTI0Hz7m7D3ZdHYU+80XL-5alyVp O States of Matter Using a phase diagram to find a phase transition temperature or pressure se the phase diagram of Substance X below to find the boiling point of X when the pressure on the liquid is 1.6 atm. pressure (atm) 32- 16- solid liquid 0. gas 100 200 temperature (K) 300 Note: your answer must be within 12.5 °C of the exact answer to be graded correct. 10 Explanation Check § Q Search J 2025 McGraw Hill LLC. All Rights Researrow_forward
- 151.2 254.8 85.9 199.6 241.4 87.6 242.5 186.4 155.8 257.1 242.9 253.3 256.0 216.6 108.7 239.0 149.7 236.4 152.1 222.7 148.7 278.2 268.7 234.4 262.7 283.2 143.6 QUESTION: Using this group of data on salt reduced tomato sauce concentration readings answer the following questions: 1. 95% Cl Confidence Interval (mmol/L) 2. [Na+] (mg/100 mL) 3. 95% Na+ Confidence Interval (mg/100 mL)arrow_forwardResults Search Results Best Free Coursehero Unloc xb Success Confirmation of Q x O Google Pas alekscgi/x/lsl.exe/1o_u-IgNslkr 7j8P3jH-IQs_pBanHhvlTCeeBZbufu BYTI0Hz7m7D3ZcHYUt80XL-5alyVpwDXM TEZayFYCavJ17dZtpxbFD0Qggd1J O States of Matter Using a phase diagram to find a phase transition temperature or pressure Gabr 3/5 he pressure above a pure sample of solid Substance X at 101. °C is lowered. At what pressure will the sample sublime? Use the phase diagram of X below to nd your answer. pressure (atm) 24- 12 solid liquid gas 200 400 temperature (K) 600 ote: your answer must be within 0.15 atm of the exact answer to be graded correct. atm Thanation Check © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center I Q Search L³ ملةarrow_forward301.7 348.9 193.7 308.6 339.5 160.6 337.7 464.7 223.5 370.5 326.6 327.5 336.1 317.9 203.8 329.8 221.9 331.7 211.7 309.6 223.4 353.7 334.6 305.6 340.0 304.3 244.7 QUESTION: Using this group of data on regular tomato sauce concentration readings answer the following questions: 1. 95% Cl Confidence Interval (mmol/L) 2. [Na+] (mg/100 mL) 3. 95% Na+ Confidence Interval (mg/100 mL)arrow_forward
- Search Results Search Results Best Free Coursehero Unlo x b Success Confirmation of Q aleks.com/alekscgi/x/sl.exe/10_u-lgNslkr7j8P3jH-IQs_pBan HhvlTCeeBZbufu BYTIOHz7m7D3ZcHYUt80XL-5alyVpwDXM TEZayFYCav States of Matter Using a phase diagram to find a phase transition temperature or pressure Use the phase diagram of Substance X below to find the temperature at which X turns to a gas, if the pressure above the solid is 3.7 atm. pressure (atm) 0. 32- 16 solid liquid gas 200 temperature (K) Note: your answer must be within 20 °C of the exact answer to be graded correct. Дос Xarrow_forwardConsider the reaction below to answer the following questions: Acetoacetic ester can be prepared by the Claisen self-condensation reaction of ethyl acetate. 1. NaOEt, EtOH H&C OCH CH3 2 H30 H3C CH2 OCH2CH3 A. Write the complete stepwise mechanism for this reaction. Show all electron flow with arrows and draw all intermediate structures. B. Ethyl acetate can be prepared from ethanol as the only organic starting material. Show all reagents and structures for all intermediates in this preparation. C. Give the structures of the ester precursors for the following Claisen condensation product and formulate the reaction. OEtarrow_forwardUse the phase diagram of Substance X below to find the temperature at which X turns to a gas, if the pressure above the solid is 3.7 atm. pressure (atm) 32 16 solid liquid gas 0 0 200 temperature (K) Note: your answer must be within 20 °C of the exact answer to be graded correct. Шос ☑ كarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY