
Concept explainers
a.
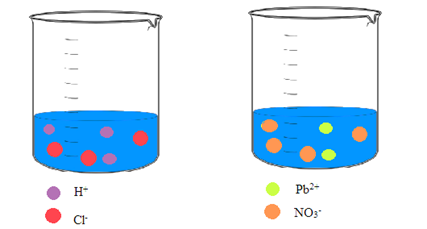
Interpretation: A picture of each solution needs to be drawn showing the ions in an aqueous solution of hydrochloric acid and an aqueous solution of lead(II) nitrate.
Concept Introduction: Ionic compounds on dissolving in water result in a solution that contains the separated ions. Due to the polar nature of water, the ions are attracted to the molecules of water.
a.
Answer to Problem 3RQ
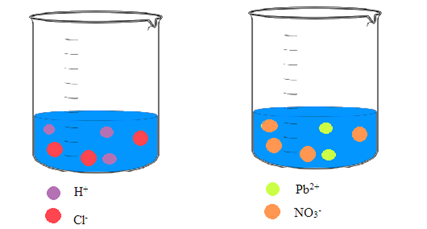
Explanation of Solution
Hydrochloric acid and lead(II) nitrate are ionic compounds that means these compounds are formed by complete transfer of electron(s). The chemical formula of hydrochloric acid and lead(II) nitrate is
From the dissociation reaction it is clear that 1 mole of
b.
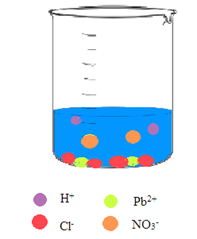
Interpretation: A picture needs to be drawn when the aqueous solution of hydrochloric acid and aqueous solution of lead(II) nitrate are mixed.
Concept Introduction: A reaction in which there is a formation of an insoluble salt due to the mixing of solutions containing soluble salts is said to be precipitation reactions.
b.
Answer to Problem 3RQ
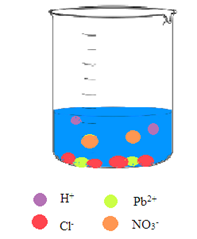
Explanation of Solution
The
So, the pictorial representation after mixing the two aqueous solutions of hydrochloric acid and lead(II) nitrate is:
c.
Interpretation: The products formed on mixing aqueous solution of hydrochloric acid and aqueous solution of lead(II) nitrate should be determined.
Concept Introduction: A reaction in which there is a formation of an insoluble salt due to the mixing of solutions containing soluble salts is said to be precipitation reactions.
c.
Answer to Problem 3RQ
The products forme are:
Solid lead chloride,
Explanation of Solution
The chemical reaction that will take place on mixing aqueous solution of hydrochloric acid and aqueous solution of lead(II) nitrate will be precipitation reaction as this reaction will lead to the formation of insoluble salt, lead chloride and aqueous nitric acid. The complete balanced reaction for the process is:
Hence, the products forme are:
Solid lead chloride,
d.
Interpretation: The net ionic equation for the reaction taken place on mixing aqueous solution of hydrochloric acid and aqueous solution of lead(II) nitrate should be determined.
Concept Introduction: The reaction that involves only the participating ions in the reaction is said to be the net ionic equation.
d.
Answer to Problem 3RQ
Explanation of Solution
The chemical reaction that will take place on mixing aqueous solution of hydrochloric acid and aqueous solution of lead(II) nitrate will be precipitation reaction as this reaction will lead to the formation of insoluble salt, lead chloride and aqueous nitric acid. The complete balanced reaction for the process is:
The total ionic equation for the above reaction is:
In the total ionic equation
Chapter 8 Solutions
World of Chemistry
- The rate coefficient of the gas-phase reaction 2 NO2 + O3 → N2O5 + O2 is 2.0x104 mol–1 dm3 s–1 at 300 K. Indicate whether the order of the reaction is 0, 1, or 2.arrow_forward8. Draw all the resonance forms for each of the following molecules or ions, and indicate the major contributor in each case, or if they are equivalent. (4.5 pts) (a) PH2 سمةarrow_forward3. Assign absolute configuration (Rors) to each chirality center. a. H Nitz C. он b. 0 H-C. C H 7 C. ་-4 917-417 refs H 1つ ८ ડુ d. Но f. -2- 01 Ho -OH 2HNarrow_forward
- How many signals do you expect in the H NMR spectrum for this molecule? Br Br Write the answer below. Also, in each of the drawing areas below is a copy of the molecule, with Hs shown. In each copy, one of the H atoms is colored red. Highlight in red all other H atoms that would contribute to the same signal as the H already highlighted red. Note for advanced students: In this question, any multiplet is counted as one signal. Number of signals in the 'H NMR spectrum. For the molecule in the top drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box at right. No additional Hs to color in top molecule For the molecule in the bottom drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box at right. No additional Hs to color in bottom moleculearrow_forwardIn the drawing area below, draw the major products of this organic reaction: 1. NaOH ? 2. CH3Br If there are no major products, because nothing much will happen to the reactant under these reaction conditions, check the box under the drawing area instead. No reaction. Click and drag to start drawing a structure. ☐ : A คarrow_forwardPredict the major products of the following organic reaction: NC Δ ? Some important Notes: • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to draw bonds carefully to show important geometric relationships between substituents. Note: if your answer contains a complicated ring structure, you must use one of the molecular fragment stamps (available in the menu at right) to enter the ring structure. You can add any substituents using the pencil tool in the usual way. Click and drag to start drawing a structure. Х аarrow_forward
- Predict the major products of this organic reaction. Be sure you use dash and wedge bonds to show stereochemistry where it's important. + ☑ OH 1. TsCl, py .... 文 P 2. t-BuO K Click and drag to start drawing a structure.arrow_forwardConsider this organic reaction: ( Draw the major products of the reaction in the drawing area below. If there won't be any major products, because this reaction won't happen at a significant rate, check the box under the drawing area instead. Click and drag to start drawing a structure. Х : а ค 1arrow_forwardIn the drawing area below, draw the major products of this organic reaction: If there are no major products, because nothing much will happen to the reactant under these reaction conditions, check the box under the drawing area instead. 1. NaH 2. CH3Br ? Click and drag to start drawing a structure. No reaction. : ☐ Narrow_forward
- + Predict the major product of the following reaction. : ☐ + ☑ ค OH H₂SO4 Click and drag to start drawing a structure.arrow_forwardConsider this organic reaction: ... OH CI Draw the major products of the reaction in the drawing area below. If there won't be any major products, because this reaction won't happen at a significant rate, check the box under the drawing area instead. ☐ No Reaction. Click and drag to start drawing a structure. : аarrow_forwardConsider the following reactants: Br Would elimination take place at a significant rate between these reactants? Note for advanced students: by significant, we mean that the rate of elimination would be greater than the rate of competing substitution reactions. yes O no If you said elimination would take place, draw the major products in the upper drawing area. If you said elimination would take place, also draw the complete mechanism for one of the major products in the lower drawing area. If there is more than one major product, you may draw the mechanism that leads to any of them. Major Products:arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





