
Concept explainers
(a)
Interpretation:
The IUPAC name of the given compound has to be written.
Concept Introduction:
Isomers: Compounds that have same molecular formula but different structural formula.
Constitutional isomers: Compounds that have same molecular formula but different connectivity (arrangement of atoms are different).
Nomenclature of organic compounds:
The naming of the organic compound is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
- The longer number of Carbon chain of a compound is identified is called parent of the compound.
- In the cyclic compounds the number of carbon involving in ring formation is called parent of the compound.
- The compound have more than one parent chains means the larger number of substitutions present in the chain is consider as a parent chain.
- The names of all substituents are arranged by alphabets to starts with lowest numbering.
- In the complex substituent having compounds the substituent name is assigned by a name each of them based on numbers going away from the parent.
(a)
Explanation of Solution
Compound (a):
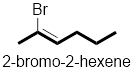
In the given compound, the longest carbon chain (highlighted with bold lines) contains six carbons and while numbering the parent chain, substituents should get the least possible number.
The presence of double bond at C-2 makes parent name as 2-hexene. The substituents Bromine atom at C-2; are arranged in alphabetical order followed by the parent name.
Therefore, the systematic name of the given compounds is ‘2-bromo-2-hexene’.
(b)
Interpretation:
The IUPAC name and configuration of the given compound has to be written.
Concept Introduction:
Nomenclature of organic compounds:
The naming of the organic compound is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
- The longer number of Carbon chain of a compound is identified this is called parent of the compound.
- In the cyclic compounds the number of carbon involving in ring formation is called parent of the compound.
- The compound have more than one parent chains means the larger number of substitutions present in the chain is consider as a parent chain.
- The names of all substituents are arranged by alphabets to starts with lowest numbering.
- In the complex substituent having compounds the substituent name is assigned by a name each of them based on numbers going away from the parent.
R and S nomenclature:
It is used to assign the molecule using CIP rules.
The CIP rules are as follows:
- Select the chiral carbon and assign the numbers according to the decreasing
atomic mass of atoms attached to it. - If the numbering follows clockwise direction then the atom is termed as R and if it follows anti-clockwise direction then atom is termed as S.
(b)
Explanation of Solution
Compound (b):
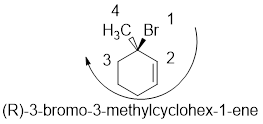
In the given compound, the longest carbon ring (highlighted with bold lines) contains six carbons and while numbering the parent chain, substituents should get the least possible number.
The presence of double bond at C-1 makes parent name is hex-1-ene. The substituents methyl located at C-3 and bromine atom at C-3; are arranged in alphabetical order followed by the parent name.
The chiral carbon and assign the numbers according to the decreasing atomic mass of atoms attached to it, follows clockwise direction then the atom is termed as R
Therefore, the systematic name of the given compounds is ‘3-bromo-3-methylcyclohex-1-ene’.
(c)
Interpretation:
The IUPAC name and configuration of the given compound has to be written.
Concept Introduction:
Nomenclature of organic compounds:
The naming of the organic compound is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
- The longer number of Carbon chain of a compound is identified this is called parent of the compound.
- In the cyclic compounds the number of carbon involving in ring formation is called parent of the compound.
- The compound have more than one parent chains means the larger number of substitutions present in the chain is consider as a parent chain.
- The names of all substituents are arranged by alphabets to starts with lowest numbering.
- In the complex substituent having compounds the substituent name is assigned by a name each of them based on numbers going away from the parent.
In a cis isomer, groups are attached on the same side of the ring.
In a trans isomer, groups are attached on the opposite side of the ring.
(c)
Explanation of Solution
Compound (c):

In the given compound, the longest carbon ring (highlighted with bold lines) contains six carbons and while numbering the parent chain, substituents should get the least possible number.
The parent name is CYCLOHEXANE. The substituents bromine atoms are at C-1and C-4; are arranged in alphabetical order followed by the parent name. Here, bromine atoms are attached on the opposite side of the ring thus ‘trans’.
Therefore, the systematic name of the given compounds is ‘trans 1,4-dibromocyclohexane’.
(d)
Interpretation:
The IUPAC name of the given compound has to be written.
Concept Introduction:
Nomenclature of organic compounds:
The naming of the organic compound is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
- The longer number of Carbon chain of a compound is identified this is called parent of the compound.
- In the cyclic compounds the number of carbon involving in ring formation is called parent of the compound.
- The compound have more than one parent chains means the larger number of substitutions present in the chain is consider as a parent chain.
- The names of all substituents are arranged by alphabets to starts with lowest numbering.
- In the complex substituent having compounds the substituent name is assigned by a name each of them based on numbers going away from the parent.
(d)
Explanation of Solution
Compound (d):

In the given compound, the longest carbon chain (highlighted with bold lines) contains four carbons and while numbering the parent chain, substituents should get the least possible number.
The parent name is butane. The substituents chlorine atom at C-1 and C-4; are arranged in alphabetical order followed by the parent name.
Therefore, the systematic name of the given compounds is ‘1,4-dichlorobutane’.
(e)
Interpretation:
The IUPAC name and configuration of the given compound has to be written.
Concept Introduction:
Nomenclature of organic compounds:
The naming of the organic compound is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
- The longer number of Carbon chain of a compound is identified this is called parent of the compound.
- In the cyclic compounds the number of carbon involving in ring formation is called parent of the compound.
- The compound have more than one parent chains means the larger number of substitutions present in the chain is consider as a parent chain.
- The names of all substituents are arranged by alphabets to starts with lowest numbering.
- In the complex substituent having compounds the substituent name is assigned by a name each of them based on numbers going away from the parent.
R and S nomenclature:
It is used to assign the molecule using CIP rules.
The CIP rules are as follows:
- Select the chiral carbon and assign the numbers according to the decreasing atomic mass of atoms attached to it.
- If the numbering follows clockwise direction then the atom is termed as R and if it follows anti-clockwise direction then atom is termed as S.
(e)
Explanation of Solution
Compound (e):
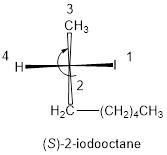
In the given compound, the longest carbon ring contains eight carbons and while numbering the parent chain, substituents should get the least possible number. The parent name is octane. The substituents iodine atom located at C-2; are arranged in alphabetical order followed by the parent name.
The numbering follows clockwise direction as shown above then it is termed as R. Least priority group is above the plane so the configuration is reversed.
Therefore, the systematic name of the given compounds is ‘(S)-2-iodooctane’.
(f)
Interpretation:
The IUPAC name and configuration of the given compound has to be written.
Concept Introduction:
Nomenclature of organic compounds:
The naming of the organic compound is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
- The longer number of Carbon chain of a compound is identified this is called parent of the compound.
- In the cyclic compounds the number of carbon involving in ring formation is called parent of the compound.
- The compound have more than one parent chains means the larger number of substitutions present in the chain is consider as a parent chain.
- The names of all substituents are arranged by alphabets to starts with lowest numbering.
- In the complex substituent having compounds the substituent name is assigned by a name each of them based on numbers going away from the parent.
R and S nomenclature:
It is used to assign the molecule using CIP rules.
The CIP rules are as follows:
- Select the chiral carbon and assign the numbers according to the decreasing atomic mass of atoms attached to it.
- If the numbering follows clockwise direction then the atom is termed as R and if it follows anti-clockwise direction then atom is termed as S.
(f)
Explanation of Solution
Compound (f):
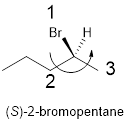
In the given compound, the longest carbon ring (highlighted with bold lines) contains five carbons and while numbering the parent chain, substituents should get the least possible number.
The parent name is hexane. The substituents bromine atom located at C-2; are arranged in alphabetical order followed by the parent name.
The chiral carbon and assign the numbers according to the decreasing atomic mass of atoms attached to it, follows anti-clockwise direction then atom is termed as S.
Therefore, the systematic name of the given compounds is ‘(S)-2-bromopentane’.
(g)
Interpretation:
The IUPAC name and configuration of the given compound has to be written.
Concept Introduction:
Nomenclature of organic compounds:
The naming of the organic compound is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
- The longer number of Carbon chain of a compound is identified this is called parent of the compound.
- In the cyclic compounds the number of carbon involving in ring formation is called parent of the compound.
- The compound have more than one parent chains means the larger number of substitutions present in the chain is consider as a parent chain.
- The names of all substituents are arranged by alphabets to starts with lowest numbering.
- The complex substituent is built by a substituent on a substituent; so called complex substituent.
- In the complex substituent having compounds the substituent name is assigned by a name each of them based on numbers going away from the parent.
R and S nomenclature:
It is used to assign the molecule using CIP rules.
The CIP rules are as follows:
- Select the chiral carbon and assign the numbers according to the decreasing atomic mass of atoms attached to it.
- If the numbering follows clockwise direction then the atom is termed as R and if it follows anti-clockwise direction then atom is termed as S.
(g)
Explanation of Solution
Compound (g):
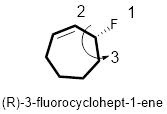
In the given compound, the longest carbon ring (highlighted with bold lines) contains seven carbons in a ring and while numbering the parent chain, substituents should get the least possible number.
The presence of double bond at C-1 on the ring makes parent name is cyclohept-1-ene. The substituents Fluorine atom located at C-3; are arranged in alphabetical order followed by the parent name.
The chiral carbon and assign the numbers according to the decreasing atomic mass of atoms attached to it, follows anti-clockwise direction then atom is termed as S. Least priority group is above the plane so the configuration is reversed. Hence, the configuration is (R).
Therefore, the systematic name of the given compounds is ‘(R)- 3-fluorocyclohept-1-ene’.
(h)
Interpretation:
The IUPAC name of the given compound has to be written.
Concept Introduction:
Nomenclature of organic compounds:
The naming of the organic compound is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
- The longer number of Carbon chain of a compound is identified this is called parent of the compound.
- In the cyclic compounds the number of carbon involving in ring formation is called parent of the compound.
- The compound have more than one parent chains means the larger number of substitutions present in the chain is consider as a parent chain.
- The names of all substituents are arranged by alphabets to starts with lowest numbering.
- The complex substituent is built by a substituent on a substituent; so called complex substituent.
- In the complex substituent having compounds the substituent name is assigned by a name each of them based on numbers going away from the parent.
(h)
Explanation of Solution
Compound (h):
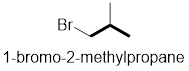
In the given compound, the longest carbon chain (highlighted with bold lines) contains three carbons and while numbering the parent chain, substituents should get the least possible number.
The parent name is Propane. The substituents methyl located at C-2 and bromine atom at C-1; are arranged in alphabetical order followed by the parent name.
Therefore, the systematic name of the given compounds is ‘1-bromo-2-methylpropane’.
(i)
Interpretation:
The IUPAC names and configuration of the given compound has to be written.
Concept Introduction:
Nomenclature of organic compounds:
The naming of the organic compound is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
- The longer number of Carbon chain of a compound is identified this is called parent of the compound.
- In the cyclic compounds the number of carbon involving in ring formation is called parent of the compound.
- The compound have more than one parent chains means the larger number of substitutions present in the chain is consider as a parent chain.
- The names of all substituents are arranged by alphabets to starts with lowest numbering.
- In the complex substituent having compounds the substituent name is assigned by a name each of them based on numbers going away from the parent.
(i)
Explanation of Solution
Compound (i):
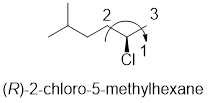
In the given compound, the longest carbon chain (highlighted with bold lines) contains six carbons and while numbering the parent chain, substituents should get the least possible number.
The parent name is hexane. The substituents methyl located at C-5 and chlorine atom at C-2; are arranged in alphabetical order followed by the parent name.
The chiral carbon and assign the numbers according to the decreasing atomic mass of atoms attached to it, follows clockwise direction then the atom is termed as R.
Therefore, the systematic name of the given compounds is ‘(R)-2-chloro-5-methylhexane’.
Want to see more full solutions like this?
Chapter 8 Solutions
Organic Chemistry
- What would be the reagents and conditions above and below the arrow that will complete the proposed acetoacetic ester synthesis? If it cannot be done efficiently, then I will choose that answer. There could be 2 or 4 reagents involved. Please provide a detailed explanation and drawings showing how it would proceed with the correct reagents.arrow_forwardFor benzene, the ∆H° of vaporization is 30.72 kJ/mol and the ∆S° of vaporization is 86.97 J/mol・K. At 1.00 atm and 228.0 K, what is the ∆G° of vaporization for benzene, in kJ/mol?arrow_forwardThe reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reaction. it is spontaneous only at High T, it is spontaneous at low T it is nonspontaneous at all T it is spontanrous at all T. it is non spontaneous only at low T.arrow_forward
- The reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reactionarrow_forwardWhich of the following has the largest standard molar entropy, S° (298.15 K) He H2 NaCl KBr Hgarrow_forwardWhich of the following is true for a particular reaction if ∆G° is -40.0 kJ/mol at 290 K and –20.0 kJ/mol at 390 K?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
