
(a)
Interpretation: The conditions need to be determined for the P-T curve for CO2at which when the temperature is increased, the solid is first converted to the liquid and then to the gaseous state.
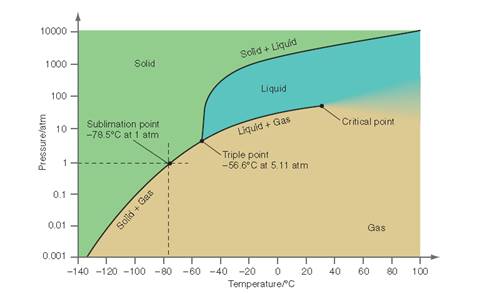
Concept Introduction: All substances can mainly exist in three phases; solid, liquid and gases. These three phases can convert into each other by the application of temperature and pressure such as by heating of solid, it converts to liquid and then gaseous
Similarly, the gases can be compressed at high pressure to liquefy. The interconversion of three phases of substance can be show on the phase diagram. A triple point indicates the value of T and P at which all the three phases of a substance that is pressure, temperature and volume coexist. At this point, the fusion, sublimation and vaporization curve intersect each other.
(b)
Interpretation: The conditions at which the interface delineating liquid and gaseous phase is observed throughout the pressure range between 6 and 65 atm needs to be determined for the below P-T curve for
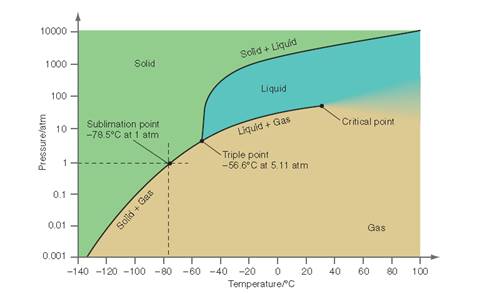
Concept Introduction: All substances can mainly exist in three phases; solid, liquid and gases. These three phases can convert into each other by the application of temperature and pressure such as by heating of solid, it converts to liquid and then gaseous state of matter.
Similarly, the gases can be compressed at high pressure to liquefy. The interconversion of three phases of substance can be show on the phase diagram. A triple point indicates the value of T and P at which all the three phases of a substance that is pressure, temperature and volume coexist. At this point, the fusion, sublimation and vaporization curve intersect each other.
(c)
Interpretation: The conditions at which the solid, liquid and gas phase coexist at equilibrium for the below P-T curve for
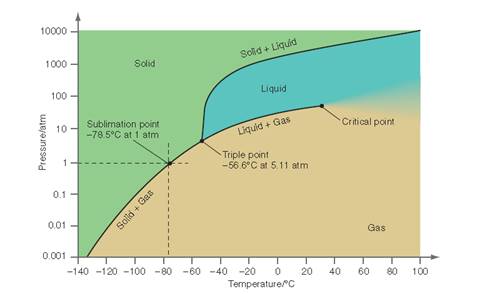
Concept Introduction: All substances can mainly exist in three phases; solid, liquid and gases. These three phases can convert into each other by the application of temperature and pressure such as by heating of solid, it converts to liquid and then gaseous state of matter.
Similarly, the gases can be compressed at high pressure to liquefy. The interconversion of three phases of substance can be show on the phase diagram. A triple point indicates the value of T and P at which all the three phases of a substance that is pressure, temperature and volume coexist. At this point, the fusion, sublimation and vaporization curve intersect each other.
(d)
Interpretation: The conditions at which only a liquid phase is observed in the pressure range from 10 to 50 atm needs to be determined for the below P-T curve for
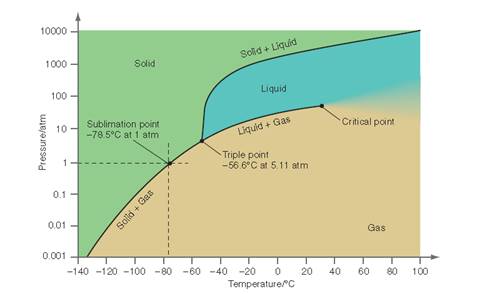
Concept Introduction: All substances can mainly exist in three phases; solid, liquid and gases. These three phases can convert into each other by the application of temperature and pressure such as by heating of solid, it converts to liquid and then gaseous state of matter.
Similarly, the gases can be compressed at high pressure to liquefy. The interconversion of three phases of substance can be show on the phase diagram. A triple point indicates the value of T and P at which all the three phases of a substance that is pressure, temperature and volume coexist. At this point, the fusion, sublimation and vaporization curve intersect each other.
(e)
Interpretation: The conditions at which an increase in the temperature from - 80 to 20 °C converts the solid to gas with no intermediate liquid phase needs to be determined.
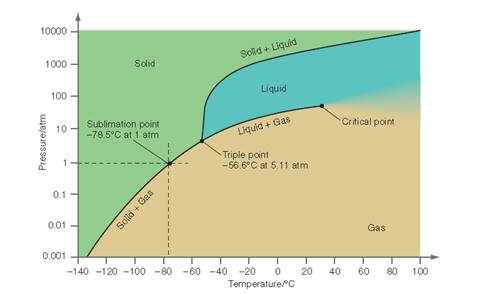
Concept Introduction: All substances can mainly exist in three phases; solid, liquid and gases. These three phases can convert into each other by the application of temperature and pressure such as by heating of solid, it converts to liquid and then gaseous state of matter.
Similarly, the gases can be compressed at high pressure to liquefy. The interconversion of three phases of substance can be show on the phase diagram. A triple point indicates the value of T and P at which all the three phases of a substance that is pressure, temperature and volume coexist. At this point, the fusion, sublimation and vaporization curve intersect each other.
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
Physical Chemistry Plus Mastering Chemistry With Etext -- Access Card Package (3rd Edition) (engel Physical Chemistry Series)
- Indicate the products obtained by mixing 2,2-dimethylpropanal with acetaldehyde and sodium ethoxide in ethanol.arrow_forwardSynthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIf possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forward
- Synthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIndicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forwardWe mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forward
- Indicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forwardIndicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forwardIndicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





