
Physical Chemistry Plus Mastering Chemistry With Etext -- Access Card Package (3rd Edition) (engel Physical Chemistry Series)
3rd Edition
ISBN: 9780321766205
Author: Thomas Engel, Philip Reid
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
Chapter 8, Problem 8.21CP
Interpretation Introduction
Interpretation: The following figure should be redrawn in the given figure indicating the four-step process d (blue arrows):
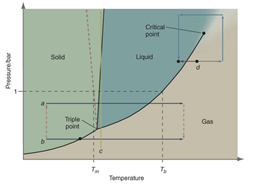
Concept introduction: A substance’s physical states under different conditions of pressure and temperature represented in a graphical manner is said to be phase diagram.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Don't use ai to answer I will report you answer
Consider a solution of 0.00304 moles of 4-nitrobenzoic acid (pKa = 3.442) dissolved in 25 mL water and titrated with 0.0991 M NaOH. Calculate the pH at the equivalence point
What is the name of the following compound?
SiMe3
Chapter 8 Solutions
Physical Chemistry Plus Mastering Chemistry With Etext -- Access Card Package (3rd Edition) (engel Physical Chemistry Series)
Ch. 8 - Prob. 8.1CPCh. 8 - Prob. 8.2CPCh. 8 - Prob. 8.3CPCh. 8 - Prob. 8.4CPCh. 8 - Prob. 8.5CPCh. 8 - Prob. 8.6CPCh. 8 - Prob. 8.7CPCh. 8 - Prob. 8.8CPCh. 8 - Prob. 8.9CPCh. 8 - Prob. 8.10CP
Ch. 8 - Prob. 8.11CPCh. 8 - Prob. 8.12CPCh. 8 - Prob. 8.13CPCh. 8 - Prob. 8.14CPCh. 8 - Prob. 8.15CPCh. 8 - Prob. 8.16CPCh. 8 - Prob. 8.17CPCh. 8 - Prob. 8.18CPCh. 8 - Prob. 8.19CPCh. 8 - Prob. 8.20CPCh. 8 - Prob. 8.21CPCh. 8 - Prob. 8.1NPCh. 8 - Prob. 8.2NPCh. 8 - Prob. 8.3NPCh. 8 - Prob. 8.4NPCh. 8 - Prob. 8.5NPCh. 8 - Prob. 8.6NPCh. 8 - Prob. 8.7NPCh. 8 - Prob. 8.8NPCh. 8 - Prob. 8.9NPCh. 8 - Prob. 8.10NPCh. 8 - Prob. 8.11NPCh. 8 - Prob. 8.12NPCh. 8 - Prob. 8.13NPCh. 8 - Prob. 8.14NPCh. 8 - Prob. 8.15NPCh. 8 - Prob. 8.16NPCh. 8 - Prob. 8.17NPCh. 8 - Prob. 8.18NPCh. 8 - Prob. 8.20NPCh. 8 - Prob. 8.21NPCh. 8 - Prob. 8.22NPCh. 8 - Prob. 8.23NPCh. 8 - Prob. 8.24NPCh. 8 - Prob. 8.25NPCh. 8 - Prob. 8.26NPCh. 8 - Prob. 8.27NPCh. 8 - Prob. 8.28NPCh. 8 - Prob. 8.29NPCh. 8 - Prob. 8.30NPCh. 8 - Prob. 8.31NPCh. 8 - Prob. 8.32NPCh. 8 - Prob. 8.33NPCh. 8 - Prob. 8.34NPCh. 8 - Prob. 8.35NPCh. 8 - Prob. 8.36NPCh. 8 - Prob. 8.37NPCh. 8 - Prob. 8.38NPCh. 8 - Prob. 8.39NPCh. 8 - Prob. 8.40NPCh. 8 - Prob. 8.41NPCh. 8 - Prob. 8.42NPCh. 8 - Prob. 8.43NPCh. 8 - Prob. 8.44NPCh. 8 - Prob. 8.45NP
Knowledge Booster
Similar questions
- K Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward7 Comment on the general features of the predicted (extremely simplified) ¹H- NMR spectrum of lycopene that is provided below. 00 6 57 PPM 3 2 1 0arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY