
Physical Chemistry Plus Mastering Chemistry With Etext -- Access Card Package (3rd Edition) (engel Physical Chemistry Series)
3rd Edition
ISBN: 9780321766205
Author: Thomas Engel, Philip Reid
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
Chapter 8, Problem 8.38NP
Interpretation Introduction
Interpretation:
The enthalpy of vaporization of n-butane needs to be deduced graphically from the given temperature and pressure data.
Concept Introduction:
Vaporization is a phase transition reaction which involves a change in the state of a substance from the liquid to the vapor phase
Enthalpy of sublimation (ΔHvap) is the amount of heat energy required for the phase conversion
The relationship between pressure (P), temperature (T) and the enthalpy change (ΔH) is given via the Clausius-Clapeyron equation:
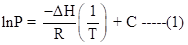
Where R = 8.314 J/mol-K and C is a constant
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Synthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.
Synthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.
Synthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.
Chapter 8 Solutions
Physical Chemistry Plus Mastering Chemistry With Etext -- Access Card Package (3rd Edition) (engel Physical Chemistry Series)
Ch. 8 - Prob. 8.1CPCh. 8 - Prob. 8.2CPCh. 8 - Prob. 8.3CPCh. 8 - Prob. 8.4CPCh. 8 - Prob. 8.5CPCh. 8 - Prob. 8.6CPCh. 8 - Prob. 8.7CPCh. 8 - Prob. 8.8CPCh. 8 - Prob. 8.9CPCh. 8 - Prob. 8.10CP
Ch. 8 - Prob. 8.11CPCh. 8 - Prob. 8.12CPCh. 8 - Prob. 8.13CPCh. 8 - Prob. 8.14CPCh. 8 - Prob. 8.15CPCh. 8 - Prob. 8.16CPCh. 8 - Prob. 8.17CPCh. 8 - Prob. 8.18CPCh. 8 - Prob. 8.19CPCh. 8 - Prob. 8.20CPCh. 8 - Prob. 8.21CPCh. 8 - Prob. 8.1NPCh. 8 - Prob. 8.2NPCh. 8 - Prob. 8.3NPCh. 8 - Prob. 8.4NPCh. 8 - Prob. 8.5NPCh. 8 - Prob. 8.6NPCh. 8 - Prob. 8.7NPCh. 8 - Prob. 8.8NPCh. 8 - Prob. 8.9NPCh. 8 - Prob. 8.10NPCh. 8 - Prob. 8.11NPCh. 8 - Prob. 8.12NPCh. 8 - Prob. 8.13NPCh. 8 - Prob. 8.14NPCh. 8 - Prob. 8.15NPCh. 8 - Prob. 8.16NPCh. 8 - Prob. 8.17NPCh. 8 - Prob. 8.18NPCh. 8 - Prob. 8.20NPCh. 8 - Prob. 8.21NPCh. 8 - Prob. 8.22NPCh. 8 - Prob. 8.23NPCh. 8 - Prob. 8.24NPCh. 8 - Prob. 8.25NPCh. 8 - Prob. 8.26NPCh. 8 - Prob. 8.27NPCh. 8 - Prob. 8.28NPCh. 8 - Prob. 8.29NPCh. 8 - Prob. 8.30NPCh. 8 - Prob. 8.31NPCh. 8 - Prob. 8.32NPCh. 8 - Prob. 8.33NPCh. 8 - Prob. 8.34NPCh. 8 - Prob. 8.35NPCh. 8 - Prob. 8.36NPCh. 8 - Prob. 8.37NPCh. 8 - Prob. 8.38NPCh. 8 - Prob. 8.39NPCh. 8 - Prob. 8.40NPCh. 8 - Prob. 8.41NPCh. 8 - Prob. 8.42NPCh. 8 - Prob. 8.43NPCh. 8 - Prob. 8.44NPCh. 8 - Prob. 8.45NP
Knowledge Booster
Similar questions
- If possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIndicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forward
- We mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forwardIndicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forward
- Indicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forwardQuestion 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forwardIndicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are mixed with sodium ethoxide in ethanol.arrow_forward
- Indicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are reacted with sodium ethoxide in ethanol.arrow_forward2,2-Dimethylpropanal and acetaldehyde are reacted with sodium ethoxide in ethanol. Indicate the products obtained.arrow_forwardAdd conditions above and below the arrow that turn the reactant below into the product below in a single transformationADS fint anditions 百 Abl res condinese NC ง Add on condtions 1.0 B H,N.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY