
Concept explainers
Interpretation:
The pH values after the addition of each proportion of the base to the acid is to be determined. Also, the titration curve needs to be drawn.
Concept introduction:
Titration curve is drawn to determine the change in pH of an acid or base with respect to the added volume of base or acid to it.
The titration curve can be drawn between a strong/weak acid and strong/weak base. The change in pH shows different patterns for different combinations of acids and bases.
Explanation of Solution
Initial pH of the analyte solution can be determined as follows:
Propanoic acid is a weak acid that forms equilibrium when dissolved in water. The equilibrium is as follows.
The amount of acid at the beginning
| Reaction | Proanoic acid | Propanoate ion | OH- |
| Initial | 0.1 | 0 | 0 |
| Change | -x | +x | +x |
| Equilibrium | (0.1-x) | x | x |
The acid dissociation constant can be represented as follows:
Solving this quadratic equation gives the amount of hydrogen ions in the solution.
On solving the only possible value of x is
Now, pH can be calculated as follows:
Addition of
Total amount of acid to be neutralized
Amount of base added
Then the ICE table after the addition of base is created in order to determine the pH of the solution using Henderson-Hasselbalch equation.
| Reaction | Propanoic acid | OH- | Propanoate ion | H+ |
| Initial | 0.0025 | 0 | 0 | 0 |
| Add | 0 | 0.0004 | ||
| Change | -0.0004 | -0.0004 | 0.0004 | 0.0004 |
| Equilibrium | 0.0021 | 0 | 0.0004 | 0.0004 |
Concentration of base after addition of acid
Concentration of ammonium ion
In the Henderson-Hasselbalch equation, the pKa is used.
Applying the Henderson-Hasselbalch equation,
Addition of
Total amount of acid to be neutralized
Amount of base added
Then the ICE table after the addition of base is created in order to determine the pH of the solution using Henderson-Hasselbalch equation.
| Reaction | Propanoic acid | OH- | Propanoate ion | H+ |
| Initial | 0.0025 | 0 | 0 | 0 |
| Add | 0 | 0.0008 | ||
| Change | -0.0008 | -0.0008 | 0.0008 | 0.0008 |
| Equilibrium | 0.0017 | 0 | 0.0008 | 0.0008 |
Concentration of acid after addition of base
Concentration of propanoate ion
Applying the Henderson-Hasselbalch equation,
Addition of
Total amount of acid to be neutralized
Amount of base added
Then the ICE table after the addition of base is created in order to determine the pH of the solution using Henderson-Hasselbalch equation.
| Reaction | Propanoic acid | OH- | Propanoate ion | H+ |
| Initial | 0.0025 | 0 | 0 | 0 |
| Add | 0 | 0.00125 | ||
| Change | -0.00125 | -0.00125 | 0.00125 | 0.00125 |
| Equilibrium | 0.00125 | 0 | 0.00125 | 0.00125 |
Concentration of acid after addition of base
Concentration of propanoate ion
Applying the Henderson-Hasselbalch equation,
Addition of
Total amount of acid to be neutralized
Amount of base added
Then the ICE table after the addition of base is created in order to determine the pH of the solution using Henderson-Hasselbalch equation.
| Reaction | Propanoic acid | H+ | Propanoate ion | OH- |
| Initial | 0.0025 | 0 | 0 | 0 |
| Add | 0 | 0.002 | ||
| Change | -0.002 | -0.002 | 0.002 | 0.002 |
| Equilibrium | 0.0005 | 0 | 0.002 | 0.002 |
Concentration of acid after addition of base
Concentration of propanoate ion
Applying the Henderson-Hasselbalch equation,
Addition of
Total amount of acid to be neutralized
Amount of base added
Then the ICE table after the addition of base is created in order to determine the pH of the solution using Henderson-Hasselbalch equation.
| Reaction | Propanoic | OH- | Propanoate | H+ |
| Initial | 0.0025 | 0 | 0 | 0 |
| Add | 0 | 0.0024 | ||
| Change | -0.0024 | -0.0024 | 0.0024 | 0.0024 |
| Equilibrium | 0.0001 | 0 | 0.0024 | 0.0024 |
Concentration of acid after addition of base
Concentration of propanoate ion
Applying the Henderson-Hasselbalch equation,
Addition of
Total amount of acid to be neutralized
Amount of base added
Then the ICE table after the addition of base is created in order to determine the pH of the solution using Henderson-Hasselbalch equation.
| Reaction | Propanoic | OH- | Propanoate | H+ |
| Initial | 0.0025 | 0 | 0 | 0 |
| Add | 0 | 0.00245 | ||
| Change | -0.00245 | -0.00245 | -0.00245 | -0.00245 |
| Equilibrium | 0.00005 | 0 | -0.00245 | -0.00245 |
Concentration of acid after addition of base
Concentration of propanoate ion
Applying the Henderson-Hasselbalch equation,
Addition of
Total amount of acid to be neutralized
Amount of base added
Then the ICE table after the addition of base is created in order to determine the pH of the solution using Henderson-Hasselbalch equation.
| Reaction | Propanoic | OH- | Propanoate | H+ |
| Initial | 0.0025 | 0 | 0 | 0 |
| Add | 0 | 0.00249 | ||
| Change | -0.00249 | -0.00249 | -0.00249 | -0.00249 |
| Equilibrium | 0.00001 | 0 | -0.00249 | -0.00249 |
Concentration of acid after addition of base
Concentration of propanoate ion
Applying the Henderson-Hasselbalch equation,
Addition of
Total amount of acid to be neutralized
Amount of base added
Then the ICE table after the addition of base is created in order to determine the pH of the solution using Henderson-Hasselbalch equation.
| Reaction | Propanoic | OH- | Propanoate | H+ |
| Initial | 0.0025 | 0 | 0 | 0 |
| Add | 0 | 0.0025 | ||
| Change | -0.0025 | -0.0025 | -0.0025 | -0.0025 |
| Equilibrium | 0.0000 | 0 | -0.0025 | -0.0025 |
Concentration of acid after addition of base
Concentration of propanoate ion
At this point, there is no excess acid or base. Therefore, the only possible reaction here is the dissociation of the conjugate acid of the propanoic acid (that is propanoate ion).
Thereafter, using the Kb value for propanoate ion, the amount of hydrogen ions in the solution can be determined to get the pH value at this point.
| Reaction | Propanoate ion | Propanoic acid | OH- |
| Initial | 0.05 | 0 | 0 |
| Change | -X | +x | +x |
| Equilibrium | (0.05-x) | x | x |
Then the pH can be calculated as follows:
On solving the equation, the only possible value of x will be:
This is the concentration of hydroxide ion. The pOH value can be calculated as follows:
Thus, pH of the solution is
Addition of
Total amount of acid to be neutralized
Amount of base added
Then the ICE table after the addition of base is created in order to determine the pH of the solution using Henderson-Hasselbalch equation.
| Reaction | Propanoic | OH- | Propanoate | H+ |
| Initial | 0.0025 | 0 | 0 | 0 |
| Add | 0 | 0.0028 | ||
| Change | -0.0025 | 0.0025 | 0 | 0 |
| Equilibrium | 0 | 0.0003 | 0 | 0 |
Concentration of hydroxide ion
Addition of
Total amount of acid to be neutralized
Amount of base added
Then the ICE table after the addition of base is created in order to determine the pH of the solution using Henderson-Hasselbalch equation.
| Reaction | Propanoic | OH- | Propanoate | H+ |
| Initial | 0.0025 | 0 | 0 | 0 |
| Add | 0 | 0.0030 | ||
| Change | -0.0025 | 0.0025 | 0 | 0 |
| Equilibrium | 0 | 0.0005 | 0 | 0 |
Concentration of hydroxide ion
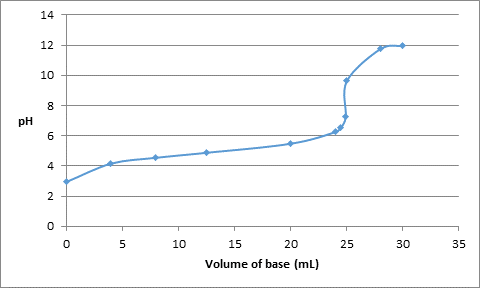
Thus, the pH values for volume of base added are as follows:
| Volume of base added (mL) | pH |
| 0.0 | 2.94 |
| 4.0 | 4.16 |
| 8.0 | 4.55 |
| 12.5 | 4.88 |
| 20.0 | 5.48 |
| 24.0 | 6.26 |
| 24.5 | 6.57 |
| 24.9 | 7.26 |
| 25.0 | 9.64 |
| 28.0 | 11.75 |
| 30.0 | 11.96 |
The titration curve can be drawn as follows:
Want to see more full solutions like this?
Chapter 8 Solutions
Chemical Principles
- 6. The equilibrium constant for the reaction 2 HBr (g) → H2(g) + Br2(g) Can be expressed by the empirical formula 11790 K In K-6.375 + 0.6415 In(T K-¹) - T Use this formula to determine A,H as a function of temperature. Calculate A,-H at 25 °C and at 100 °C.arrow_forward3. Nitrosyl chloride, NOCI, decomposes according to 2 NOCI (g) → 2 NO(g) + Cl2(g) Assuming that we start with no moles of NOCl (g) and no NO(g) or Cl2(g), derive an expression for Kp in terms of the equilibrium value of the extent of reaction, Seq, and the pressure, P. Given that K₂ = 2.00 × 10-4, calculate Seq/ of 29/no when P = 0.080 bar. What is the new value по ƒª/ at equilibrium when P = 0.160 bar? Is this result in accord with Le Châtelier's Principle?arrow_forwardConsider the following chemical equilibrium: 2SO2(g) + O2(g) = 2SO3(g) • Write the equilibrium constant expression for this reaction. Now compare it to the equilibrium constant expression for the related reaction: • . 1 SO2(g) + O2(g) = SO3(g) 2 How do these two equilibrium expressions differ? What important principle about the dependence of equilibrium constants on the stoichiometry of a reaction can you learn from this comparison?arrow_forward
- Given Kp for 2 reactions. Find the Kp for the following reaction: BrCl(g)+ 1/2 I2(g) ->IBr(g) + 1/2 Cl2(g)arrow_forwardFor a certain gas-phase reaction at constant pressure, the equilibrium constant Kp is observed to double when the temperature increases from 300 K to 400 K. Calculate the enthalpy change of the reaction, Ah, using this information.arrow_forwardHydrogen bonding in water plays a key role in its physical properties. Assume that the energy required to break a hydrogen bond is approximately 8 kJ/mol. Consider a simplified two-state model where a "formed" hydrogen bond is in the ground state and a "broken" bond is in the excited state. Using this model: • Calculate the fraction of broken hydrogen bonds at T = 300 K, and also at T = 273 K and T = 373 K. • At what temperature would approximately 50% of the hydrogen bonds be broken? • What does your result imply about the accuracy or limitations of the two-state model in describing hydrogen bonding in water? Finally, applying your understanding: • Would you expect it to be easier or harder to vaporize water at higher temperatures? Why? If you were to hang wet laundry outside, would it dry more quickly on a warm summer day or on a cold winter day, assuming humidity is constant?arrow_forward
- (3 pts) Use the Kapustinskii equation to calculate the lattice enthalpy for MgBr2 anddiscuss any differences between this result and that from #4.arrow_forward(3 pts) Silver metal adopts a fcc unit cell structure and has an atomic radius of 144 pm. Fromthis information, calculate the density of silver. Show all work.arrow_forward4. (3 pts) From the information below, determine the lattice enthalpy for MgBr2. Show all work. AH/(kJ mol-¹) Sublimation of Mg(s) +148 lonization of Mg(g) +2187 to Mg2+(g) Vaporization of Br₂(1) +31 Dissociation of Br,(g) +193 Electron gain by Br(g) -331 Formation of MgBr₂(s) -524arrow_forward
- 1. (4 pts-2 pts each part) Consider the crystal structures of NaCl, ZnS, and CsCl (not necessarily shown in this order). a. For one of the three compounds, justify that the unit cell is consistent with stoichiometry of the compound. b. In each of the crystal structures, the cations reside in certain holes in the anions' packing structures. For each compound, what type of holes are occupied by the cations and explain why those particular types of holes are preferred.arrow_forward(2 pts) What do you expect to happen in a Na2O crystal if a Cl− ion replaces one of the O2−ions in the lattice?arrow_forward(2 pts) WSe2 is an ionic compound semiconductor that can be made to be p-type or n-type.What must happen to the chemical composition for it to be p-type? What must happen tothe chemical composition for it to be n-type?arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning





