
Concept explainers
Consider the following
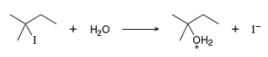
a. Draw a mechanism for this reaction using curved arrows.
b. Draw an energy diagram. Label the axes, the reactants, products,
c. Draw the structure of any transition states.
d. What is the rate equation for this reaction?
e. What happens to the reaction rate in each of the following instances? [1] The leaving group is changed from
(a)
Interpretation: The mechanism of the given reaction is to be drawn by the use of curved arrows.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron rich chemical species that contains negative charge or lone pair of electrons are known as a nucleophile. In a nucleophilic substitution reaction, nucleophile takes the position of leaving group by attacking the electron deficient carbon atom.
Answer to Problem 7.58P
The mechanism of the given reaction is shown in Figure 1.
Explanation of Solution
The structure of the given alkyl halide shows that carbon atom, on which bromine is present, is bonded to three other carbon atoms. Hence, the bromine atom is bonded to tertiary carbon atom and the given alkyl halide is
In
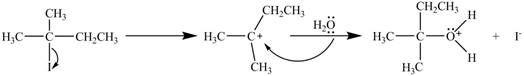
Figure 1
The mechanism of the given reaction is shown in Figure 1.
(b)
Interpretation: The energy diagram is to be drawn. The axes, reactants, products,
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The graphical representation of chemical reaction in which x-axis represents energy of the reaction and y-axis represents the reaction coordinate is called energy profile diagram.
Answer to Problem 7.58P
The energy diagram of the given reaction is shown in Figure 2.
Explanation of Solution
In
The energy of products is equal to the energy of reactants. Therefore, the energy diagram of the given reaction is,
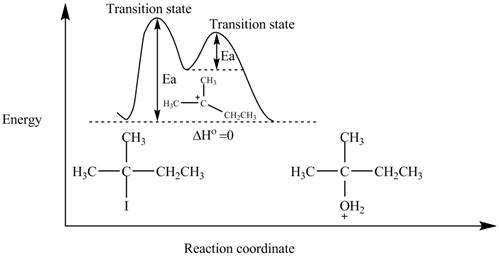
Figure 2
The x-axes of the energy diagram represent the energy of reactants and products and y-axes represent the reaction coordinate.
The energy diagram of the given reaction is shown in Figure 2.
(c)
Interpretation: The structure of the transition state is to be drawn.
Concept introduction: In
Answer to Problem 7.58P
The structure of the transition states is shown in Figure 3.
Explanation of Solution
In
Therefore, the transition states of the given reaction are,
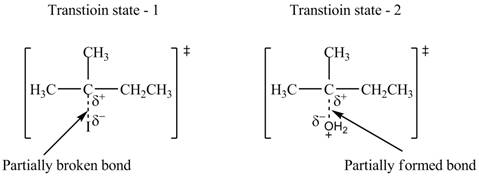
Figure 3
The structure of the transition states is shown in Figure 3.
(d)
Interpretation: The rate equation of the given reaction is to be predicted.
Concept introduction: The rate of
The rate of
Answer to Problem 7.58P
The rate equation of the given reaction is,
Explanation of Solution
The rate of
The rate of
The alkyl halide of given reaction is
Therefore, the rate equation of given reaction is,
The rate equation of the given reaction is,
(e)
Interpretation: The change that occurs to the reaction rate in given instances is to be stated.
Concept introduction: The rate of
The rate of
Answer to Problem 7.58P
The change that occurs to the reaction rate in given instances is,
[1] The rate of the reaction will decrease.
[2] The rate of the reaction will decrease.
[3] The rate of the reaction will decrease.
[4] The rate of the reaction remains unchanged.
[5] The rate of the reaction increases by five times.
Explanation of Solution
[1]
The tertiary halide undergoes nucleophilic substitution by
[2]
The polar protic solvent favors
[3]
The tertiary halide undergoes nucleophilic substitution by
[4]
The rate of
The rate of
According the given statement, the concentration of
Therefore, the rate of the reaction remains unchanged when the concentration of
[5]
The rate of
The rate of
According the given statement, the concentration of
Therefore, the rate of the reaction increases by five times when the concentration
The change that occurs to the reaction rate in given instances is,
[1] The rate of the reaction will decrease.
[2] The rate of the reaction will decrease.
[3] The rate of the reaction will decrease.
[4] The rate of the reaction remains unchanged.
[5] The rate of the reaction increases by five times.
Want to see more full solutions like this?
Chapter 7 Solutions
Organic Chemistry
Additional Science Textbook Solutions
Organic Chemistry
Concepts of Genetics (12th Edition)
Biological Science (6th Edition)
Chemistry: Structure and Properties (2nd Edition)
Organic Chemistry (8th Edition)
The Cosmic Perspective (8th Edition)
- 2. Use Hess's law to calculate the AH (in kJ) for: rxn CIF(g) + F2(g) → CIF 3 (1) using the following information: 2CIF(g) + O2(g) → Cl₂O(g) + OF 2(g) AH = 167.5 kJ ΔΗ 2F2 (g) + O2(g) → 2 OF 2(g) 2C1F3 (1) + 202(g) → Cl₂O(g) + 3 OF 2(g) о = = -43.5 kJ AH = 394.1kJarrow_forwardci Draw the major product(s) of the following reactions: (3 pts) CH3 HNO3/H2SO4 HNO3/ H2SO4 OCH3 (1 pts)arrow_forwardProvide the product for the reactionarrow_forward
- What is the net ionic equation for the reaction between tin(IV) sulfide and nitric acid?arrow_forwardThe combustion of 28.8 g of NH3 consumes exactly _____ g of O2. 4 NH3 + 7 O2 ----> 4 NO2 + 6 H2Oarrow_forwardWhat is the molecular formula of the bond-line structure shown below OH HO ○ C14H12O2 ○ C16H14O2 ○ C16H12O2 O C14H14O2arrow_forward
- Check all molecules that are acids on the list below. H2CO3 HC2H3O2 C6H5NH2 HNO3 NH3arrow_forwardFrom the given compound, choose the proton that best fits each given description. a CH2 CH 2 Cl b с CH2 F Most shielded: (Choose one) Least shielded: (Choose one) Highest chemical shift: (Choose one) Lowest chemical shift: (Choose one) ×arrow_forwardConsider this molecule: How many H atoms are in this molecule? How many different signals could be found in its 1H NMR spectrum? Note: A multiplet is considered one signal.arrow_forward
- For each of the given mass spectrum data, identify whether the compound contains chlorine, bromine, or neither. Compound m/z of M* peak m/z of M + 2 peak ratio of M+ : M + 2 peak Which element is present? A 122 no M + 2 peak not applicable (Choose one) B 78 80 3:1 (Choose one) C 227 229 1:1 (Choose one)arrow_forwardShow transformation from reactant to product, step by step. *see imagearrow_forwardCheck the box if the molecule contains the listed item. *See imagearrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
