
Concept explainers
(a)
Interpretation:
The Lewis formula of
Concept Introduction:
Lewis Structure: A Lewis structure shows a covalent bond as pair of electrons shared between two atoms.
Procedure to write Lewis formulas:
- 1) The symbols of the atoms that are bonded together in the molecule next to one another are arranged.
- 2) The total number of valence electrons in the molecule is calculated by adding the number of valence electrons for all the atoms in the molecules. If the species is an ion, then the charge of ion into account by adding electrons, if it is a negative ion or subtracting electrons if it is a positive ion.
- 3) A two-electron covalent bond is represented by placing a line between the atoms, which are assumed to be bonded to each other.
- 4) The remaining valence electrons as lone pairs about each atom are arranged so that the octet rule is satisfied for each other.
Formal charge (F.C): The charges that assigned to each atom in a molecule or ion by a set of arbitrary rules and don not actually represent the actual charges on the atoms are called as formal charges.
The formal charge is calculated using the formula,
The Lewis structure with zero formal charge or least separated formal charges is the preferred structure of the molecule.
(a)
Explanation of Solution
The total number of valence electrons in
Number of valence electrons in nitrogen=
Number of valence electrons in fluorine=
Since there is a positive charge present in ion, one electron is subtracted.
The total number of valence electrons is thirty-two.
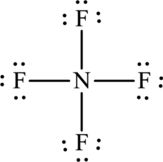
One nitrogen atom forms four bonds with fluorine that is eight electrons are used to form bonds and the remaining twenty-four atoms are used to satisfy the octet rule of fluorine atoms.
The Lewis formula is,
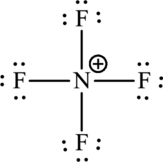
The formal charge for each atom is calculated as,
Formal charge on fluorine=
Formula charge on nitrogen=
The Lewis formula is,
(b)
Interpretation:
The Lewis formula of
Concept Introduction:
Refer to part (a).
(b)
Explanation of Solution
The total number of valence electrons in
Number of valence electrons in chlorine=
Number of valence electrons in fluorine=
Since there is a positive charge present in ion, one electron is subtracted.
The total number of valence electrons is thirty-four.
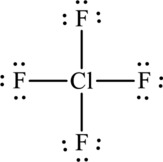
One chlorine atom forms four bonds with fluorine that is eight electrons are used to form bonds and the remaining twenty-six atoms are used to satisfy the octet rule of fluorine atoms.
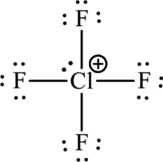
The Lewis formula is,
There are two unassigned electrons and the two unassigned electrons can be added to a chlorine atom. As chlorine is present in the third period, it can expand the octet rule.
The Lewis structure becomes,
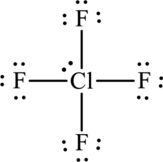
The formal charge for each atom is calculated as,
Formal charge on fluorine=
Formula charge on chlorine=
The Lewis formula is,
(c)
Interpretation:
The Lewis formula of
Concept Introduction:
Refer to part (a).
(c)
Explanation of Solution
The total number of valence electrons in
Number of valence electrons in phosphorus=
Number of valence electrons in hydrogen=
Since there is a positive charge present in ion, one electron is subtracted.
The total number of valence electrons is eight.
One phosphorus atom forms four bonds with hydrogen that is eight electrons are used to form bonds.
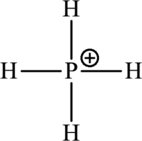
The Lewis formula is,
The formal charge for each atom is calculated as,
Formal charge on phosphorus=
Formula charge on hydrogen=
The Lewis formula is,
(d)
Interpretation:
The Lewis formula of
Concept Introduction:
Refer to part (a).
(d)
Explanation of Solution
The total number of valence electrons in
Number of valence electrons in arsenic=
Number of valence electrons in fluorine=
Since there is a negative charge present in ion, one electron is added.
The total number of valence electrons is forty-eight.
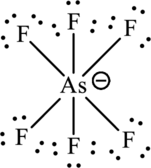
One arsenic atom forms six bonds with fluorine that is twelve electrons are used to form that bonds and the remaining thirty-six are used to satisfy the octet rule of fluorine atoms.
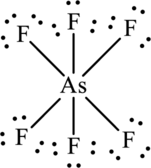
The Lewis formula is,
The formal charge for each atom is calculated as,
Formal charge on arsenic=
Formula charge on fluorine=
The Lewis formula is,
(e)
Interpretation:
The Lewis formula of
Concept Introduction:
Refer to part (a).
(e)
Explanation of Solution
The total number of valence electrons in
Number of valence electrons in bromine=
Number of valence electrons in fluorine=
Since there is a negative charge present in ion, one electron is added.
The total number of valence electrons is thirty-six.
One bromine atom forms four bonds with fluorine that is eight electrons are used to form that bonds and the remaining twenty-eight electrons are used to satisfy the octet rule of fluorine atoms.
The Lewis formula is,
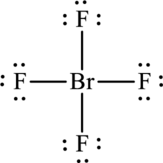
There are only thirty-two valence electrons and four electrons are left unassigned. The unassigned electrons are added to bromine atom. As bromine atom is present in the fourth period, it can expand the octet rule.
The Lewis structure becomes,
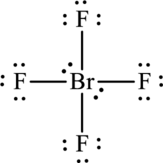
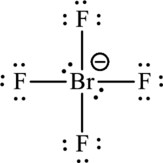
The formal charge for each atom is calculated as,
Formal charge on bromine=
Formula charge on fluorine=
The Lewis formula is,
Want to see more full solutions like this?
Chapter 7 Solutions
General Chemistry
- Find a mole of : H2SO4 (15 ml 3.0 M) NaOH ( 18 mL 2.5 M) Acetic anhydride (3.0 mL)arrow_forwardFor the mechanism, show everything, lone paires, charges and arrow pleasearrow_forwardmolecule 0= OH ☐ ☐ type of molecule (check all that apply) fatty acid monoglyceride diglyceride triglyceride saturated unsaturated monounsaturated ☐ polyunsaturated ☐ ☐ ☐ ☐ ☐ 010 0 0 0 0 0 0 ☐ ☐ ☐ ☐☐☐☐ U omega-3 omega-6 fatty acid monoglyceride diglyceride triglyceride saturated unsaturated monounsaturated polyunsaturated omega-3 omega-6 fatty acid monoglyceride diglyceride triglyceride saturated unsaturated monounsaturated polyunsaturated omega-3 omega-6 OH OHarrow_forward
- '☐ : ☑ ด Suppose an alien life form has DNA just like human DNA remain the same.) - except that the alien DNA is made from deoxyarabinose instead of deoxyribose. (All other ingredients Draw the structure of a nucleotide containing thymine from which the alien DNA would be assembled. Note: be sure to draw the molecule as it would exist at physiological pH. Click and drag to start drawing a structure.arrow_forwardPredict the products of the following biochemical reaction: CH2 CH-O + 3 KOH CH2-0 In particular, draw the structure of the product or products P in the drawing area below. If there are no products, because this reaction won't happen, check the No reaction box under the drawing area. Note: if there is more than one product, you can draw them in any arrangement you like. Also, just draw the structure of each product. You don't have to draw the complete right-hand side of the equation, including stoichiometric coefficients. No reaction Click and drag to start drawing a structure. : 5 èarrow_forwardAssign these spectrumarrow_forward
- If I have 30% H2O2, indicate how to prepare a 6% H2O2 solution.arrow_forward7) 8) FCI II -C-C-C=C-C || Br Br || -C=C-Br -CEC-C-C- 10) 11) F Br i OH مله 12) Br i 13) 14) 15) CH3CHFCHFC=CH C(OH)Br2CHF(CH2)4CH2CH3 CH3(CH2)3CH=CH(CH2)2CH3arrow_forwardName 1) 3-fluoro, 1-butene 2) 2-heptene 2,3-difluoro- 1-pentene 4) 6-iodo,4-methyl- 2-decyne 5) 4,4-dibromo- 1,2-butandiol Complete structural formula F -C=C-C-C- Line formula Condensed structural formula N F CH2=CHCHFCH3arrow_forward
- 1. Part 1: Naming Organic Compounds он H₁C-C-CH3 CH3 Br CI CI 2. Br-CH-CH-CH₂ H₂C-CH-C= -CH-CH2-CH3 3. HC-CH-CH-C-OH 5. H₂C-CH-CH₂-OH 7. OH 4. CH CH₂-CH₂ 6. сно CH-CH-CH-CH₂-CH₂ H₁₂C-CH-CH-CH-CH₁₂-CH₁₂ 8. OHarrow_forward11 Organic Chemistry Organic Nomenclature Practice Name/Functional Group n-butane Formula Structural Formula (1) C4tt10 H3C C- (2) CH3CH2CH2 CH 3 H₂ -CH3 Н2 name & functional group (1) and (2) OH H₁₂C Н2 name only (1) and (2) name only (1) and (2) H₁C - = - CH₂ Н2 HC=C-C CH3arrow_forwardUnder aqueous basic conditions, nitriles will react to form a neutral organic intermediate 1 that has an N atom in it first, and then they will continue to react to form the final product 2: NC H₂O он- H₂O 1 2 OH Draw the missing intermediate 1 and the final product 2 in the box below. You can draw the two structures in any arrangement you like. Click and drag to start drawing a structure.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





