
(a)
Interpretation:
To draw the given structure corresponding to each name.
Concept introduction:
Answer to Problem 7.45P
Given the structure name is isopropyl bromide
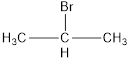
Explanation of Solution
To write the corresponding structure. To work backward from a name to the structure.
First find the parent name and draw it according to the number of carbons. Use the suffix to identify the
Then arrange the number of carbon in the chain. Finally add the substituent to the appropriate carbon.
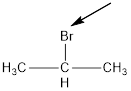
The bromide is in the middle carbon make it into a isopropyl.
Thus given the structure name is isopropyl bromide

(b)
Interpretation:
To draw the given structure corresponding to each name.
Concept introduction:
Alkyl halides are organic molecules that contains a halogen atom X bonded to sp3 hybridized carbon atom. Alkyl halides are classified as primary (1°), secondary (2°), and tertiary (3°) depending on the number of carbons bonded to the carbon with the halogen. An alkyl halide is named as an alkane with a halogen substituent-that is, as a halo alkane. To name a halogen substituent, change the -ine ending of the name of the halogen to the suffix -o (chlorine-chloro).
Answer to Problem 7.45P
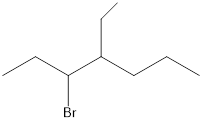
Given the structure name is 3-bromo-4-ethylheptane
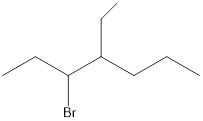
Explanation of Solution
To write the corresponding structure. To work backward from a name to the structure.
First find the parent name and draw it according to the number of carbons. Use the suffix to identify the functional group.
Then arrange the number of carbon in the chain. Finally add the substituent to the appropriate carbon.
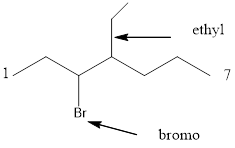
Starting from first carbon the bromide is in the third carbon and ethyl is in fourth carbon, and there are total of seven carbon so 3-bromo-4-ethylheptane.
Thus given the structure name is 3-bromo-4-ethylheptane
(c)
Interpretation:
To draw the given structure corresponding to each name.
Concept introduction:
Alkyl halides are organic molecules that contains a halogen atom X bonded to sp3 hybridized carbon atom. Alkyl halides are classified as primary (1°), secondary (2°), and tertiary (3°) depending on the number of carbons bonded to the carbon with the halogen. An alkyl halide is named as an alkane with a halogen substituent-that is, as a halo alkane. To name a halogen substituent, change the -ine ending of the name of the halogen to the suffix -o (chlorine-chloro).
Answer to Problem 7.45P
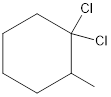
Given the structure name is 1, 1-dichloro-2-methylcyclohexane
Explanation of Solution
To write the corresponding structure. To work backward from a name to the structure.
First find the parent name and draw it according to the number of carbons. Use the suffix to identify the functional group.
Then arrange the number of carbon in the chain. Finally add the substituent to the appropriate carbon.
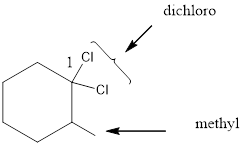
Starting from first carbon the two chlorine is in the first carbon and methyl is in second carbon, and there are total of six carbon and ring so 1, 1-dichloro-2-methylcyclohexane.
Thus Given the structure name is 1, 1-dichloro-2-methylcyclohexane

(d)
Interpretation:
To draw the given structure corresponding to each name.
Concept introduction:
Alkyl halides are organic molecules that contains a halogen atom X bonded to sp3 hybridized carbon atom. Alkyl halides are classified as primary (1°), secondary (2°), and tertiary (3°) depending on the number of carbons bonded to the carbon with the halogen. An alkyl halide is named as an alkane with a halogen substituent-that is, as a halo alkane. To name a halogen substituent, change the -ine ending of the name of the halogen to the suffix -o (chlorine-chloro).
Answer to Problem 7.45P
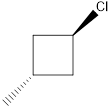
Given the structure name is trans-1-chloro-3-iodocyclobutane
Explanation of Solution
To write the corresponding structure. To work backward from a name to the structure.
First find the parent name and draw it according to the number of carbons. Use the suffix to identify the functional group.
Then arrange the number of carbon in the chain. Finally add the substituent to the appropriate carbon.
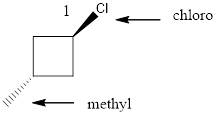
Starting from first carbon the chlorine is in the first carbon and methyl is in third carbon, and there are total of four carbon and ring so trans-1-chloro-3-iodocyclobutane.
Thus Given the structure name is trans-1-chloro-3-iodocyclobutane

(e)
Interpretation:
To draw the given structure corresponding to each name.
Concept introduction:
Alkyl halides are organic molecules that contains a halogen atom X bonded to sp3 hybridized carbon atom. Alkyl halides are classified as primary (1°), secondary (2°), and tertiary (3°) depending on the number of carbons bonded to the carbon with the halogen. An alkyl halide is named as an alkane with a halogen substituent-that is, as a halo alkane. To name a halogen substituent, change the -ine ending of the name of the halogen to the suffix -o (chlorine-chloro).
Answer to Problem 7.45P
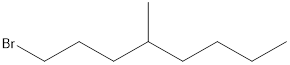
Given the structure name is 1-bromo-4-ethyl-3-fluorooctane
Explanation of Solution
To write the corresponding structure. To work backward from a name to the structure.
First find the parent name and draw it according to the number of carbons. Use the suffix to identify the functional group.
Then arrange the number of carbon in the chain. Finally add the substituent to the appropriate carbon.
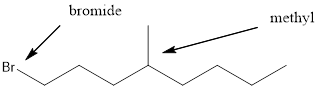
Starting from first carbon the bromide is in the first carbon and methyl is in fourth carbon, and there are total of eight carbon so 1-bromo-4-ethyl-3-fluorooctane.
Thus Given the structure name is 1-bromo-4-ethyl-3-fluorooctane

(f)
Interpretation:
To draw the given structure corresponding to each name.
Concept introduction:
Alkyl halides are organic molecules that contains a halogen atom X bonded to sp3 hybridized carbon atom. Alkyl halides are classified as primary (1°), secondary (2°), and tertiary (3°) depending on the number of carbons bonded to the carbon with the halogen. An alkyl halide is named as an alkane with a halogen substituent-that is, as a halo alkane. To name a halogen substituent, change the -ine ending of the name of the halogen to the suffix -o (chlorine-chloro).
Answer to Problem 7.45P
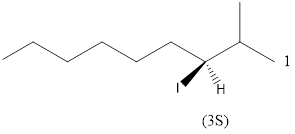
Given the structure name is (3S)-3-iodo-2-methylnonane
Explanation of Solution
To write the corresponding structure. To work backward from a name to the structure.
First find the parent name and draw it according to the number of carbons. Use the suffix to identify the functional group.
Then arrange the number of carbon in the chain. Finally add the substituent to the appropriate carbon.
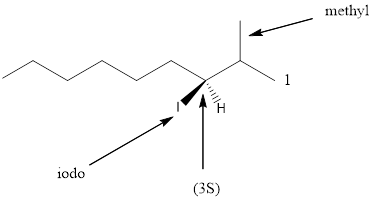
Starting from first carbon the iodo is in the third carbon and methyl is in second carbon, and there are total of nine carbon so (3S)-3-iodo-2-methylnonane.
Thus Given the structure name is (3S)-3-iodo-2-methylnonane

(g)
Interpretation:
To draw the given structure corresponding to each name.
Concept introduction:
Alkyl halides are organic molecules that contains a halogen atom X bonded to sp3 hybridized carbon atom. Alkyl halides are classified as primary (1°), secondary (2°), and tertiary (3°) depending on the number of carbons bonded to the carbon with the halogen. An alkyl halide is named as an alkane with a halogen substituent-that is, as a halo alkane. To name a halogen substituent, change the -ine ending of the name of the halogen to the suffix -o (chlorine-chloro).
Answer to Problem 7.45P
Given the structure name is (1 R, 2R)-trans-1-bromo-2-chlorocyclohexane
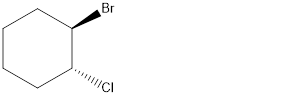
Explanation of Solution
To write the corresponding structure. To work backward from a name to the structure.
First find the parent name and draw it according to the number of carbons. Use the suffix to identify the functional group.
Then arrange the number of carbon in the chain. Finally add the substituent to the appropriate carbon.
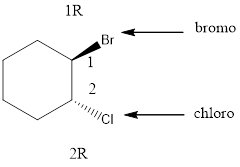
Starting from first carbon the chloro and bromo is in the first carbon and there are total of six carbon so (1 R, 2R)-trans-1-bromo-2-chlorocyclohexane.
Thus Given the structure name is (1 R, 2R)-trans-1-bromo-2-chlorocyclohexane
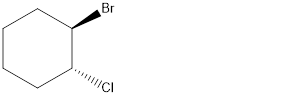
(h)
Interpretation:
To draw the given structure corresponding to each name.
Concept introduction:
Alkyl halides are organic molecules that contains a halogen atom X bonded to sp3 hybridized carbon atom. Alkyl halides are classified as primary (1°), secondary (2°), and tertiary (3°) depending on the number of carbons bonded to the carbon with the halogen. An alkyl halide is named as an alkane with a halogen substituent-that is, as a halo alkane. To name a halogen substituent, change the -ine ending of the name of the halogen to the suffix -o (chlorine-chloro).
Answer to Problem 7.45P
Given the structure name is (5R)-4, 4, 5-trichloro-3, 3-dimethyldecane
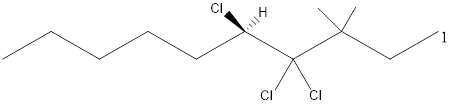
Explanation of Solution
To write the corresponding structure. To work backward from a name to the structure.
First find the parent name and draw it according to the number of carbons. Use the suffix to identify the functional group.
Then arrange the number of carbon in the chain. Finally add the substituent to the appropriate carbon.
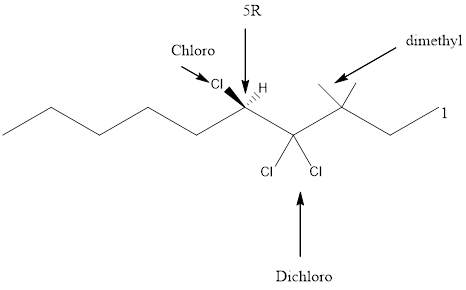
Starting from first carbon the chloro is in the fourth and fifth carbon and there are total of ten carbons so (5R)-4, 4, 5-trichloro-3, 3-dimethyldecane.
Thus Given the structure name is (5R)-4, 4, 5-trichloro-3, 3-dimethyldecane

Want to see more full solutions like this?
Chapter 7 Solutions
Organic Chemistry
- What is the product of the following reaction? Please include a detailed explanation of what is happening in this question. Include a drawing showing how the reagent is reacting with the catalyst to produce the correct product. The correct answer is IV.arrow_forwardPlease complete the reactions, thank youarrow_forwardConsider the synthesis. What is compound Y? Please explain what is happening in this question. Provide a detailed explanation and a drawing to show how the compound Y creates the product. The correct answer is D.arrow_forward
- What would be the major product of the following reaction? Please include a detailed explanation of what is happening in this question. Include steps and a drawing to show this reaction proceeds and how the final product is formed. The correct answer is B. I put answer D and I don't really understand what is going on in the question.arrow_forwardWhat is the product of the following reaction? Please explain what is happening in this question. Provide a detailed explanation and a drawing showing how the reagent is reacting with the catalysts to product the correct product. The correct answer is B.arrow_forwardWhat is the missing intermediate 1 and the final product 2. Please include a detailed explanation explaining the steps of malonic ester synthesis. Please include drawings of the intermediate and how it occurs and how the final product is former.arrow_forward
- What would be the reagents and conditions above and below the arrow that will complete the proposed acetoacetic ester synthesis? If it cannot be done efficiently, then I will choose that answer. There could be 2 or 4 reagents involved. Please provide a detailed explanation and drawings showing how it would proceed with the correct reagents.arrow_forwardFor benzene, the ∆H° of vaporization is 30.72 kJ/mol and the ∆S° of vaporization is 86.97 J/mol・K. At 1.00 atm and 228.0 K, what is the ∆G° of vaporization for benzene, in kJ/mol?arrow_forwardThe reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reaction. it is spontaneous only at High T, it is spontaneous at low T it is nonspontaneous at all T it is spontanrous at all T. it is non spontaneous only at low T.arrow_forward
- The reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reactionarrow_forwardWhich of the following has the largest standard molar entropy, S° (298.15 K) He H2 NaCl KBr Hgarrow_forwardWhich of the following is true for a particular reaction if ∆G° is -40.0 kJ/mol at 290 K and –20.0 kJ/mol at 390 K?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





