
The following diagram shows a simpli?ed version of how a refrigerator works:
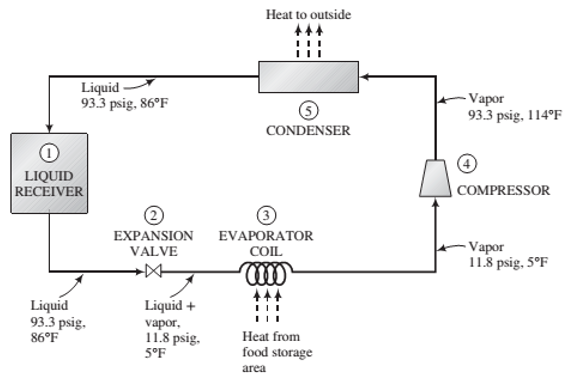
In a liquid receiver 1, a liquid refrigerant (any one of a number of halogenated hydrocarbons such as CCl2F3) is contained at high pressure and temperature. The liquid passes through an expansion valve 2, where it ?ashes to a low pressure, cooling to its boiling point at this pressure and partially evaporating. The liquid—vap0r mixture passes though an evaporator coil 3. Air from the food storage area circulates over the coil, and the heat absorbed by the evaporating refrigerant in the coil causes the air to cool. The cold refrigerant vapor emerging from the coil passes to a compressor 4, where it is brought back to a high pressure and in the process is raised to a high temperature. The hot vapor then passes through a condenser 5, where it is cooled and condensed at constant pressure. The air that absorbs the heat given up by the condensing ?uid is discharged outside the refrigerator, and the lique?ed refrigerant returns to the liquid receiver.
Suppose Refrigerant R−12 (the standard name for CCI2F2 undergoes this cycle at a circulation rate of 40 lbm/min, with the temperatures and pressures at the different points of the cycle being those shown on the ?ow diagram.
Saturated Fluid: 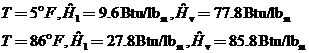
Superheated Vapor: 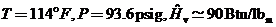
(a) Suppose the expansion valve operates adiabatically and 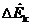 is negligible. Use an energy balance about the valve to calculate the fraction of the refrigerant that evaporates in this stage of the process.
is negligible. Use an energy balance about the valve to calculate the fraction of the refrigerant that evaporates in this stage of the process.
(b) Calculate the rate in Btu/min at which heat is transferred to the refrigerant that evaporates in the coil. (This is the useful cooling done in the system.)
(e) lf the heat loss in the condenser is 2500 Btu/min, how much horsepower must the compressor deliver to the system? (Use an overall energy balance to solve this problem.)
(d) You have just given a “What do engineers do?" talk at a middle-school career day, and one of the students in the audience asks if you can explain how a refrigerator works. Try to do it in terms that a bright l2-year-old student could understand.
(e) The manufacture of R−12 in the United States was banned as a result of the Montreal Protocol. Why?
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
ELEM.PRIN.OF CHEM.PROCESS-ACCESS
Additional Engineering Textbook Solutions
Web Development and Design Foundations with HTML5 (8th Edition)
Database Concepts (8th Edition)
Java How to Program, Early Objects (11th Edition) (Deitel: How to Program)
Computer Science: An Overview (13th Edition) (What's New in Computer Science)
Degarmo's Materials And Processes In Manufacturing
Starting Out with Java: From Control Structures through Data Structures (4th Edition) (What's New in Computer Science)
- Considering the molar flux as estimated by the Whitman two-film theory, show the relationship between the mass transfer coefficients based on concentration, and mol fraction gradients, kc and ky, respectively, is given by: ky = Ckc, where C is the total concentration. do not use chatgpt please, i did not understan from it thats why i paid for bartlebyarrow_forwardConsidering the molar flux as estimated by the Whitman two-film theory, show the relationship between the mass transfer coefficients based on concentration, and mol fraction gradients, kc and ky, respectively, is given by: ky = Ckc, where C is the total concentration. please do not use chatgpt, i did not understand from it that is why i paid for this.arrow_forwardWe have a feed that is a binary mixture of methanol and water (55.0 mol% methanol) that is sent to a system of two flash drums hooked together. The vapor from the first drum is cooled, which partially condenses the vapor, and then is fed to the second flash drum. Both drums operate at a pressure of 1.0 atm and are adiabatic. The feed rate to the first drum is 1000.0 kmol/h. We desire a liquid product from the first drum that is 30.0 mol% methanol (x1 = 0.300). The second drum operates at a fraction vaporized of (V/F)2 = 0.250. The equilibrium data are in Table 2-8. Find the following for the first drum: y1, T1, (V/F)1, and vapor flow rate V1. Find the following for the second drum: y2, x2, T2, and vapor flow rate V2.arrow_forward
- Show that the overall mass transfer coefficient, Ky, can be related to the individual gas and liquid film mass transfer coefficients, ky and kx, respectively, by the following equation: 1 K y = 1 + m kk y xarrow_forwardUse the approach given to solvearrow_forwardAntoine constants for vapor pressure for n-pentane and n-hexane are listed in Table 2-3. a. Predict the vapor pressure at 0.0°C for pure n-pentane. b. Predict the boiling point of pure n-pentane at 3.0 atm pressure. c. Predict the boiling pressure if pure n-pentane is boiling at 0.0°C. d. At a pressure of 500.0 mm Hg and temperature of 30.0°C, predict the K values for n-pentane and n-hexane using Raoult’s law. e. If T = 30.0°C and p = 500.0 mm Hg, determine the mole fractions in the liquid and vapor phases of an equilibrium mixture of n-pentane and n-hexane. f. 1.0 moles of a mixture that is 75.0 mol% n-pentane and 25.0 mol% n-hexane is placed in a closed chamber. The pressure is adjusted to 500.0 mm Hg, and the temperature to 30.0°C. The vapor and liquid mole fractions were found in part e. How many moles of liquid and moles of vapor are there at equilibrium? g. If 1.0 mol/min of a mixture that is 75.0 mol% n-pentane and 25.0 mol% n-hexane is fed continuously to an equilibrium flash…arrow_forward
- A 40 mol % ethanol 60 mol % water mixture at 60 °C and 1 atm is heated. Using Figure 2-3 answer the following:a. At what temperature does the mixture first begin to boil? What is the composition of the first bubble of vapor?b. At what temperature would it stop boiling (assume no material is removed)? What is the composition of the last droplet of liquid?c. At 82 °C, what fraction is liquid? d. When 90% has been vaporized, what is the temperature, and what are the liquid and vapor compositions?arrow_forwardIs a component with a lower vapor pressure more or less volatile than a component with a higher vapor pressure? Briefly explain.arrow_forward3.3. Use the following crude assay data with crude API of 36 to estimate cut vol%, critical properties and molecular weight for Light Naphtha (90- 190 °F) and Kerosene (380-520 °F). In addition, calculate the fractions of paraffins, naphthenes and aromatics in the two cuts. ASTM D86 (°F) Volume % Cum vol% SG 86 0.0 0.0 122 0.5 0.5 0.6700 167 1.2 1.7 0.6750 212 1.6 3.3 0.7220 257 2.7 6.0 0.7480 302 3.1 9.1 0.7650 347 3.9 13.0 0.7780 392 4.7 17.7 0.7890 437 5.7 23.4 0.8010 482 8.0 31.4 0.8140 527 10.7 42.1 0.8250 584 5.0 47.1 0.8450 636 10.0 57.1 0.8540 689 7.8 64.9 0.8630 742 7.0 71.9 0.8640 794 6.5 78.4 0.8890 20 8 002 09310 Iarrow_forward
- Problem 1) A fractional factorial design has been used to study on the effect of 3 parameters including adsorbent concentration (300 and 500 ppm), pH value (4 and 10) and reaction time (6 and 12 hours) on the adsorption capacity of a composite for removing of methylene blue from a wastewater. If the results obtained for two repetitions of the tests are according to the following table, it is desirable: A) Design Resolution Y₁ Y₂ Run (mg/g) (mg/g) B) Drawing graphs of the effect of each parameter on the adsorption capacity 1 28 26 2 36 34 C) Analysis of interactions 3 18 20 D) Calculate the effects percentage of each parameter and error 4 32 30 E) Determining the optimum conditions to achieve the highest adsorption capacityarrow_forwardThermophysical Properties of Petroleum Fractions and Crude Ofls 67 3.4. A gas oil has the following TBP distillation data Volume % TBP (°C) 0 216 10 243 30 268 50 284 70 304 90 318 95 327 100 334 It also has an average boiling point of 280 °C and an average density of 0.850 g/cm³. (a) Split this gas oil fraction into five pseudo-components. Calculate T., Pc and w for each pseudo-component. (b) Calculate T, Pc and w for the whole gas oil fraction. (c) Calculate the enthalpy of this gas oil fraction at 400 °C using the Lee- Kessler correlation with a reference state of ideal gas at 273.15 K.arrow_forward3.3. Use the following crude assay data with crude API of 36 to estimate cut vol%, critical properties and molecular weight for Light Naphtha (90- 190 °F) and Kerosene (380-520 °F). In addition, calculate the fractions of paraffins, naphthenes and aromatics in the two cuts. ASTM D86 (°F) Volume % Cum vol% SG 86 0.0 0.0 122 0.5 0.5 0.6700 167 1.2 1.7 0.6750 212 1.6 3.3 0.7220 257 2.7 6.0 0.7480 302 3.1 9.1 0.7650 347 3.9 13.0 0.7780 392 4.7 17.7 0.7890 437 5.7 23.4 0.8010 482 8.0 31.4 0.8140 527 10.7 42.1 0.8250 584 5.0 47.1 0.8450 636 10.0 57.1 0.8540 689 7.8 64.9 0.8630 742 7.0 71.9 0.8640 794 6.5 78.4 0.8890 20.8 99.2 0.9310arrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





