
Concept explainers
(a)
Interpretation:
The hybridization of each carbon atom in following structure should be determined.
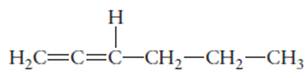
Concept Introduction:
The process of the mixing of orbitals having similar symmetry and energy to results equal number of orbitals is known as hybridization.
The formed orbitals are known as hybrid orbitals.
Carbon belongs to group 4 of the periodic table and its
The electronic configuration of carbon is:
In the hybridization, carbon uses 2s and 2p orbitals.
For example:
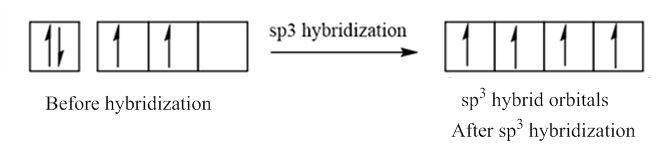
In case of sp3 hybridization, one 2s and three 2p orbitals of carbon combine to form four sp3 hybrid orbitals.
The electronic configuration of carbon before and after sp3 hybridization is:
(b)
Interpretation:
The hybridization of each carbon atom in following structure should be determined.
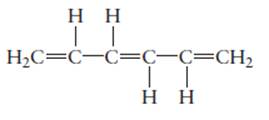
Concept Introduction:
The process of the mixing of orbitals having similar symmetry and energy to results equal number of orbitals is known as hybridization.
The formed orbitals are known as hybrid orbitals.
Carbon belongs to group 4 of the periodic table and its atomic number is 6.
The electronic configuration of carbon is:
In the hybridization, carbon uses 2s and 2p orbitals.
For example:
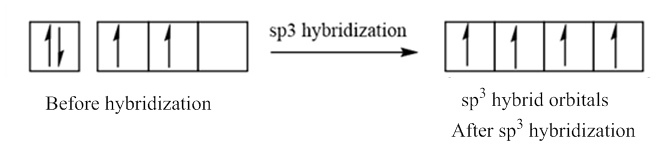
In case of sp3 hybridization, one 2s and three 2p orbitals of carbon combine to form four sp3 hybrid orbitals.
The electronic configuration of carbon before and after sp3 hybridization is:
(c)
Interpretation:
The hybridization of each carbon atom in following structure should be determined.
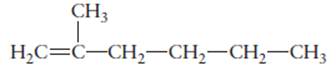
Concept Introduction:
The process of the mixing of orbitals having similar symmetry and energy to results equal number of orbitals is known as hybridization.
The formed orbitals are known as hybrid orbitals.
Carbon belongs to group 4 of the periodic table and its atomic number is 6.
The electronic configuration of carbon is:
In the hybridization, carbon uses 2s and 2p orbitals.
For example:
In case of sp3 hybridization, one 2s and three 2p orbitals of carbon combine to form four sp3 hybrid orbitals.
The electronic configuration of carbon before and after sp3 hybridization is:
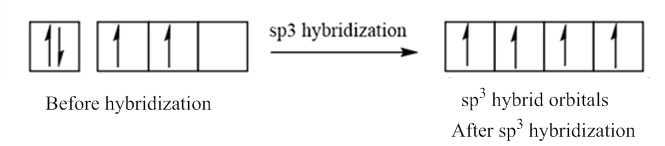
(d)
Interpretation:
The hybridization of each carbon atom in following structure should be determined.
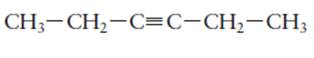
Concept Introduction:
The process of the mixing of orbitals having similar symmetry and energy to results equal number of orbitals is known as hybridization.
The formed orbitals are known as hybrid orbitals.
Carbon belongs to group 4 of the periodic table and its atomic number is 6.
The electronic configuration of carbon is:
In the hybridization, carbon uses 2s and 2p orbitals.
For example:
In case of sp3 hybridization, one 2s and three 2p orbitals of carbon combine to form four sp3 hybrid orbitals.
The electronic configuration of carbon before and after sp3 hybridization is:
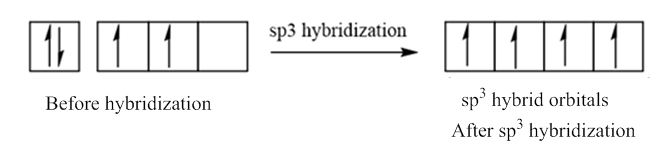
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Student Solutions Manual for Oxtoby/Gillis/Butler's Principles of Modern Chemistry, 8th
- Explain how substitutions at the 5-position of barbituric acid increase the compound's lipophilicity.arrow_forwardExplain how substitutions at the 5-position of phenobarbital increase the compound's lipophilicity.arrow_forwardName an interesting derivative of barbituric acid, describing its structure.arrow_forward
- Briefly describe the synthesis mechanism of barbituric acid from the condensation of urea with a β-diketone.arrow_forwardGiven the hydrazones indicated, draw the structures of the enamines that can be formed. Indicate the most stable enamine (explain). C6H5 C6H5 H C6H5 Harrow_forward4. Propose a Synthesis for the molecule below. You may use any starting materials containing 6 carbons or less (reagents that aren't incorporated into the final molecule such as PhзP do not count towards this total, and the starting material can have whatever non-carbon functional groups you want), and any of the reactions you have learned so far in organic chemistry I, II, and III. Your final answer should show each step separately, with intermediates and conditions clearly drawn.arrow_forward
- Indicate the importance of the indole ring. Find a representative example and list 5 structures.arrow_forwardΌΗ 1) V2 CO 3 or Nalt In منهarrow_forward6. The equilibrium constant for the reaction 2 HBr (g) → H2(g) + Br2(g) Can be expressed by the empirical formula 11790 K In K-6.375 + 0.6415 In(T K-¹) - T Use this formula to determine A,H as a function of temperature. Calculate A,-H at 25 °C and at 100 °C.arrow_forward
- 3. Nitrosyl chloride, NOCI, decomposes according to 2 NOCI (g) → 2 NO(g) + Cl2(g) Assuming that we start with no moles of NOCl (g) and no NO(g) or Cl2(g), derive an expression for Kp in terms of the equilibrium value of the extent of reaction, Seq, and the pressure, P. Given that K₂ = 2.00 × 10-4, calculate Seq/ of 29/no when P = 0.080 bar. What is the new value по ƒª/ at equilibrium when P = 0.160 bar? Is this result in accord with Le Châtelier's Principle?arrow_forwardConsider the following chemical equilibrium: 2SO2(g) + O2(g) = 2SO3(g) • Write the equilibrium constant expression for this reaction. Now compare it to the equilibrium constant expression for the related reaction: • . 1 SO2(g) + O2(g) = SO3(g) 2 How do these two equilibrium expressions differ? What important principle about the dependence of equilibrium constants on the stoichiometry of a reaction can you learn from this comparison?arrow_forwardGiven Kp for 2 reactions. Find the Kp for the following reaction: BrCl(g)+ 1/2 I2(g) ->IBr(g) + 1/2 Cl2(g)arrow_forward
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStaxChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStaxChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





