
Concept explainers
(a)
Interpretation:
The name of the following molecule should be determined.
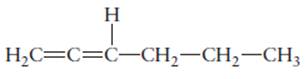
Concept Introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons.
Rules of naming
- First choose the longest continuous chain of carbon atoms having double bond known as parent chain.
- The numbering of parent chain should be done in a way that the double bond and substituents get the lowest number.
- The root name of the carbon chain is same as in case of
alkanes , but “−ane” ending is replaced by “−ene” - The appropriate name should be given to every alkyl group and denote its position on the parent chain with the number.
- The alkyl groups are written in alphabetical order.
(b)
Interpretation:
The name of the following molecule should be determined.
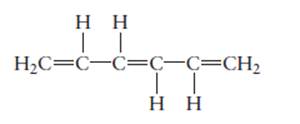
Concept Introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons. Unsaturated hydrocarbon having double bond is known as alkene having general molecular formula
Rules of naming alkenes are:
- First choose the longest continuous chain of carbon atoms having double bond known as parent chain.
- The numbering of parent chain should be done in a way that the double bond and substituents get the lowest number.
- The root name of the carbon chain is same as in case of alkanes, but “−ane” ending is replaced by “−ene”
- The appropriate name should be given to every alkyl group and denote its position on the parent chain with the number.
- The alkyl groups are written in alphabetical order.
(c)
Interpretation:
The name of the following molecule should be determined.
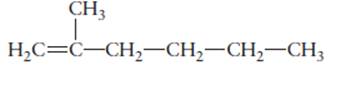
Concept Introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons. Unsaturated hydrocarbon having double bond is known as alkene having general molecular formula
Rules of naming alkenes are:
- First choose the longest continuous chain of carbon atoms having double bond known as parent chain.
- The numbering of parent chain should be done in a way that the double bond and substituents get the lowest number.
- The root name of the carbon chain is same as in case of alkanes, but “−ane” ending is replaced by “−ene”
- The appropriate name should be given to every alkyl group and denote its position on the parent chain with the number.
- The alkyl groups are written in alphabetical order.
(d)
Interpretation:
The name of the following molecule should be determined.
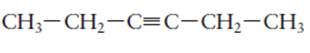
Concept Introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons. Unsaturated hydrocarbon having double bond is known as alkene having general molecular formula
Rules of naming alkynes are:
1. First choose the longest continuous chain of carbon atoms having triple bond known as parent chain.
2. The numbering of parent chain should be done in a way that the triple bond and substituents get the lowest number.
3. The root name of the carbon chain is same as in case of alkanes, but “−ane” ending is replaced by “−yne”
4. The appropriate name should be given to every alkyl group and denote its position on the parent chain with the number.
5. The alkyl groups are written in alphabetical order.
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Student Solutions Manual for Oxtoby/Gillis/Butler's Principles of Modern Chemistry, 8th
- Complete the mechanism for the E1 reaction below by following the directions written above each of the five boxes. Be sure to include lone pair electrons and nonzero formal charges. 2nd attempt 1 Provide the missing curved arrow notation. E+ RUDDA 1st attempt Feedback See Periodic Table See Hint Iir See Periodic Table See Hintarrow_forwardHeating an alcohol in the presence of sulfuric or phosphoric acid will cause a dehydration to occur: the removal of the elements of water from a molecule, forming an alkene. The reaction usually follows an E1 mechanism. The SN1 pathway is suppressed by using a strong acid whose conjugate base is a poor nucleophile. Further, heating the reaction mixture causes a greater increase in the rate of E1 compared to the rate of Sy1. 3rd attempt h Draw curved arrow(s) to show how the alcohol reacts with phosphoric acid. TH © 1 0 0 +1% # 2nd attempt Feedback H Ju See Periodic Table See Hint H Jud See Periodic Table See Hintarrow_forwardPart 2 (0.5 point) 0- Draw the major organic product with the correct geometry. 10 1: 70000 х く 1st attempt Part 1 (0.5 point) Feedback Please draw all four bonds at chiral centers. P See Periodic Table See Hintarrow_forward
- Heating an alcohol in the presence of sulfuric or phosphoric acid will cause a dehydration to occur: the removal of the elements of water from a molecule, forming an alkene. The reaction usually follows an E1 mechanism. The SN1 pathway is suppressed by using a strong acid whose conjugate base is a poor nucleophile. Further, heating the reaction mixture causes a greater increase in the rate of E1 compared to the rate of S№1. 2nd attempt 0 See Periodic Table See Hint Draw the organic intermediate from the first step (no byproducts) and draw curved arrow(s) to show how it reacts. TH +11: 1st attempt Feedback H H H C F F See Periodic Table See Hintarrow_forwardThis molecule undergoes an E1 mechanism when stirred in methanol. 3rd attempt CH₂OH CH₂OH 6148 O See Periodic Table. See Hint Draw 3 chemical species including formal charges and lone pairs of electrons. Add the missing curved arrow notation. H N O O SA 3 Br Iarrow_forwardComplete the mechanism for the E1 reaction below by following the directions written above each of the five boxes. Be sure to include lone pair electrons and nonzero formal charges. 1st attempt Y 0 + Provide the missing curved arrow notation. 01: See Periodic Table See Hint H C Br Iarrow_forward
- Please help answer number 2. Thanks in advance.arrow_forwardHow do I explain this? Thank you!arrow_forwardWhen an unknown amine reacts with an unknown acid chloride, an amide with a molecular mass of 163 g/mol (M* = 163 m/z) is formed. In the infrared spectrum, important absorptions appear at 1661, 750 and 690 cm. The 13C NMR and DEPT spectra are provided. Draw the structure of the product as the resonance contributor lacking any formal charges. 13C NMR DEPT 90 200 160 120 80 40 0 200 160 120 80 40 0 DEPT 135 T 200 160 120 80 40 0 Draw the unknown amide. Select Dow Templates More Fragearrow_forward
- Identify the unknown compound from its IR and proton NMR spectra. C4H6O: 'H NMR: 82.43 (1H, t, J = 2 Hz); 8 3.41 (3H, s); 8 4.10 (2H, d, J = 2 Hz) IR: 2125, 3300 cm¹ The C4H6O compound liberates a gas when treated with C2H5 MgBr. Draw the unknown compound. Select Draw с H Templates Morearrow_forwardPlease help with number 6 I got a negative number could that be right?arrow_forward1,4-Dimethyl-1,3-cyclohexadiene can undergo 1,2- or 1,4-addition with hydrogen halides. (a) 1,2-Addition i. Draw the carbocation intermediate(s) formed during the 1,2-addition of hydrobromic acid to 1,4-dimethyl-1,3-cyclohexadiene. ii. What is the major 1,2-addition product formed during the reaction in (i)? (b) 1,4-Addition i. Draw the carbocation intermediate(s) formed during the 1,4-addition of hydrobromic acid to 1,4-dimethyl-1,3-cyclohexadiene. ii. What is the major 1,4-addition product formed from the reaction in (i)? (c) What is the kinetic product from the reaction of one mole of hydrobromic acid with 1,4-dimethyl-1,3-cyclohexadiene? Explain your reasoning. (d) What is the thermodynamic product from the reaction of one mole of hydrobro-mic acid with 1,4-dimethyl-1,3-cyclohexadiene? Explain your reasoning. (e) What major product will result when 1,4-dimethyl-1,3-cyclohexadiene is treated with one mole of hydrobromic acid at - 78 deg * C ? Explain your reasoning.arrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax





