
Interpretation:
The ground state electronic configuration of selenium is to be obtained by using the Aufbau principle.
Concept introduction:
Aufbau Principle states that every electron occupies the orbitals in the increasing order of their energy.
The four steps for writing the ground state electronic configuration of an element are shown below:
An electron will enter the lowest energy orbital.
A maximum of two electrons can occupy each orbital.
In a degenerate orbital, the electrons do not pair until all the orbitals are occupied with at least one electron.
Electrons occupy the orbitals in the following order:
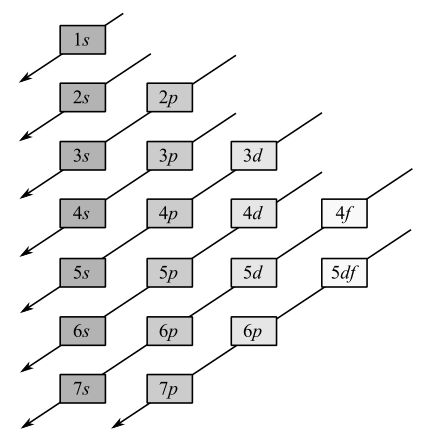
Noble gas core is used to represent electron configuration of all the elements except hydrogen and helium.
Noble gas core is an abbreviation in an atom’s electron configuration. It replaces the electron configuration of noble gas element most recently precede the element.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Chemistry
- V Consider this step in a radical reaction: Br: ? What type of step is this? Check all that apply. Draw the products of the step on the right-hand side of the drawing area below. If more than one set of products is possible, draw any set. Also, draw the mechanism arrows on the left-hand side of the drawing area to show how this happens. ⚫ionization termination initialization neutralization none of the abc Explanation Check 80 Ο F3 F1 F2 2 F4 01 % do5 $ 94 #3 X 5 C MacBook Air 25 F5 F6 66 ©2025 ˇ F7 29 & 7 8arrow_forwardShow how to convert ethyl benzene to (a) 2,5-dichlorobenzoic acid and (b) 2,4-dichlorobenzoic acid.arrow_forwardno aiarrow_forward
- Polymers may be composed of thousands of monomers. Draw three repeat units (trimer) of the polymer formed in this reaction. Assume there are hydrogen atoms there are hydrogen atoms on the two ends of the trimer. Ignore inorganic byproducts.arrow_forwardDraw a tetramer if this alternating copolymer pleasearrow_forwardDraw the monomers required to synthesize this condensation polymer.arrow_forward
- Draw the monomers required to synthesize this condensation polymer.arrow_forward8:44 PM Sun Apr 13 Earn Freecash.com O Measurement and Matter =1 Setting up a unit conversion 110 Eddie says... ✰ www-awu.aleks.com A student sets up the following equation to convert a measurement. (The ? stands for a number the student is going to calculate.) Fill in the missing part of this equation. Note: your answer should be in the form of one or more fractions multiplied together. (- 4 J kJ -7.0 × 10 ☐ = ? mmol.°C mol °C x10 μ Explanation Check □·□ torox.io Grey Hill LLC. All Rightsarrow_forwardPolymers may be composed of thousands of monomers. Draw three repeat units (trimer) of the polymer formed in this reaction. Assume there are hydrogen atoms there are hydrogen atoms on the two ends of the trimer. Ignore inorganic byproducts please.arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





