
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6, Problem 6.52P
As we have seen in this chapter, carbon-carbon double bonds are electron-rich regions that are attacked by electrophiles (e.g., HBr); they are not attacked by nucleophiles (e.g., diethylamine).
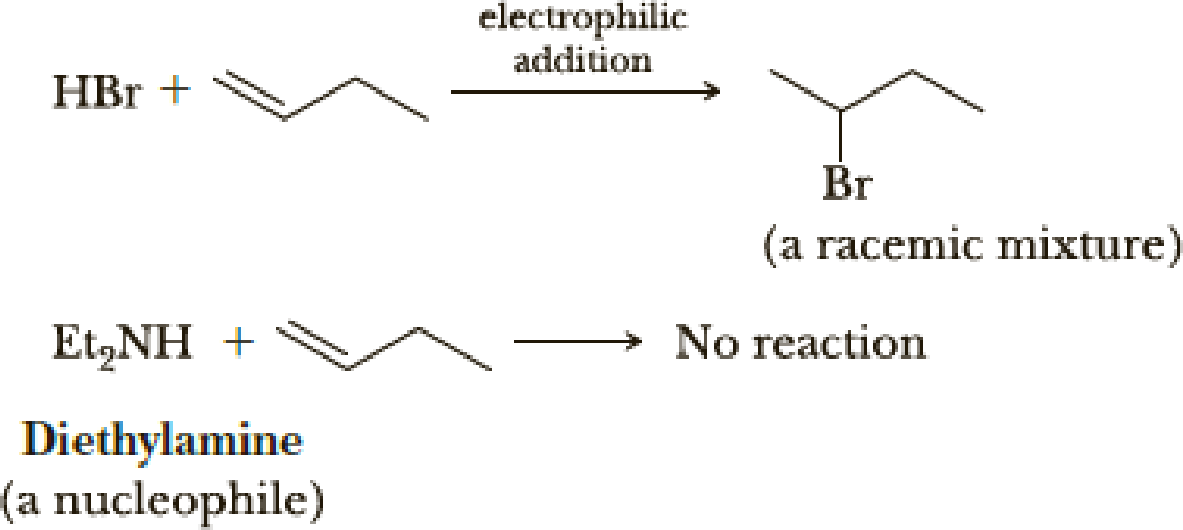
However, when the carbon-carbon double bond has a carbonyl group adjacent to it, the double bond reacts readily with nucleophiles by nucleophilic addition (Section 19.8).
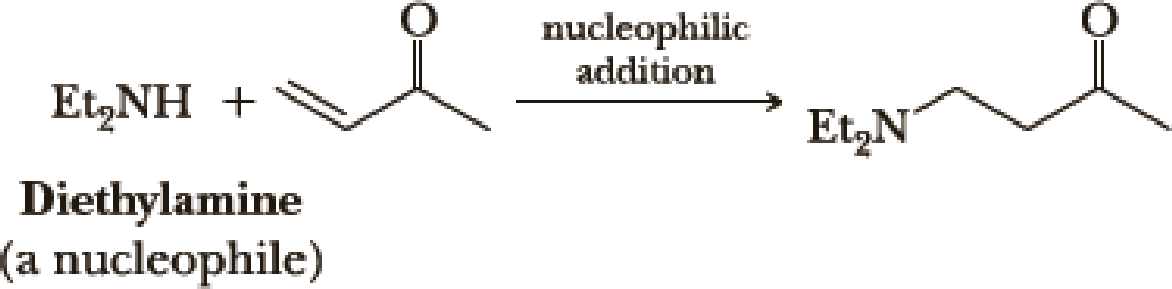
Account for the fact that nucleophiles add to a carbon-carbon double bond adjacent to a carbonyl group and account for the regiochemistry of the reaction.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Write the amididation reaction mechanism of a-aminophenol and acetic acid to produce acetaminophen
For the condensation reaction between Alamine and histamine, please help me write the amididation reaction mechanism. Then write the three letter code for the product of the reaction, then write the one letter code for the product of the reaction. 
How to draw the reaction mechasnism below
Chapter 6 Solutions
Organic Chemistry
Ch. 6.2 - Using the BDE values from Appendix 3, calculate...Ch. 6.3 - Name and draw a structural formula for the product...Ch. 6.3 - Prob. 6.3PCh. 6.3 - Propose a mechanism for the addition of HI to...Ch. 6.3 - Prob. 6.5PCh. 6.3 - Propose a mechanism for the acid-catalyzed...Ch. 6.3 - The acid-catalyzed hydration of...Ch. 6.3 - Complete these reactions. (a) (b)Ch. 6.3 - Draw the structure of the chlorohydrin formed by...Ch. 6.4 - Draw structural formulas for the alkene that gives...
Ch. 6.5 - Prob. 6.11PCh. 6.5 - Prob. 6.12PCh. 6.5 - What alkene with the molecular formula C6H12, when...Ch. 6 - Prob. 6.15PCh. 6 - Prob. 6.16PCh. 6 - Predict the organic product(s) of the reaction of...Ch. 6 - Prob. 6.18PCh. 6 - Prob. 6.20PCh. 6 - Draw a structural formula for an alkene with the...Ch. 6 - Account for the fact that addition of HCl to...Ch. 6 - Account for the fact that treating propenoic acid...Ch. 6 - Draw a structural formula for the alkene with the...Ch. 6 - Draw the alternative chair conformations for the...Ch. 6 - Draw a structural formula for the cycloalkene with...Ch. 6 - Reaction of this bicycloalkene with bromine in...Ch. 6 - Terpin, prepared commercially by the...Ch. 6 - Propose a mechanism for this reaction and account...Ch. 6 - Treating 2-methylpropene with methanol in the...Ch. 6 - When 2-pentene is treated with Cl2 in methanol,...Ch. 6 - Treating cyclohexene with HBr in the presence of...Ch. 6 - Propose a mechanism for this reaction. 1-Pentane...Ch. 6 - Treating 4-penten-1-ol with bromine in water forms...Ch. 6 - Prob. 6.35PCh. 6 - Prob. 6.36PCh. 6 - Reaction of -pinene with borane followed by...Ch. 6 - Write structural formulas for the major organic...Ch. 6 - Draw the structural formula of the alkene that...Ch. 6 - Consider the following reaction. (a) Draw a...Ch. 6 - Prob. 6.42PCh. 6 - Prob. 6.43PCh. 6 - Show how to convert ethylene to these compounds....Ch. 6 - Show how to convert cyclopentene into these...Ch. 6 - Prob. 6.46PCh. 6 - Describe the stereochemistry of the bromohydrin...Ch. 6 - Prob. 6.49PCh. 6 - Treating 1,3-butadiene with 1 mole of HBr gives a...Ch. 6 - In this chapter, we studied the mechanism of the...Ch. 6 - As we have seen in this chapter, carbon-carbon...Ch. 6 - Prob. 6.53PCh. 6 - Prob. 6.54P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Name the following molecules with IUpacarrow_forwardWhat is the molecular orbital for cyclopropenyl anion and is it aromatic, antiaromatic or nonaromatic?arrow_forwardUsing the chart describe the change from cystine to tyrosine and its impact on the protein. Using the chart describe the change from histidine to aspartic acid and its impact on the protein.arrow_forward
- How to get the predicted product of this reaction belowarrow_forwardPlease help me fill out the chart then using the chart describe the change from cystine to tyrosine and its impact on the protein. Then using the chart describe the change from histidine to aspartic acid.arrow_forwardWrite the Esterification reaction mechanism for acetic acid, and one propanol to make propanol ethanoate (molecule that gives peas its odor in flavor)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
Enzymes - Effect of cofactors on enzyme; Author: Tutorials Point (India) Ltd;https://www.youtube.com/watch?v=AkAbIwxyUs4;License: Standard YouTube License, CC-BY
Enzyme Catalysis Part-I; Author: NPTEL-NOC IITM;https://www.youtube.com/watch?v=aZE740JWZuQ;License: Standard Youtube License