
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 6, Problem 6.36P
Interpretation Introduction
Interpretation: The mechanism for given reaction has to be proposed.
Concept introduction:
Electrophilic addition reaction is an addition reaction where in a
Carbocation: The carbon ion that bears a positive charge on it.
Carbocation stability order:
The general carbocation stability order is as follows,
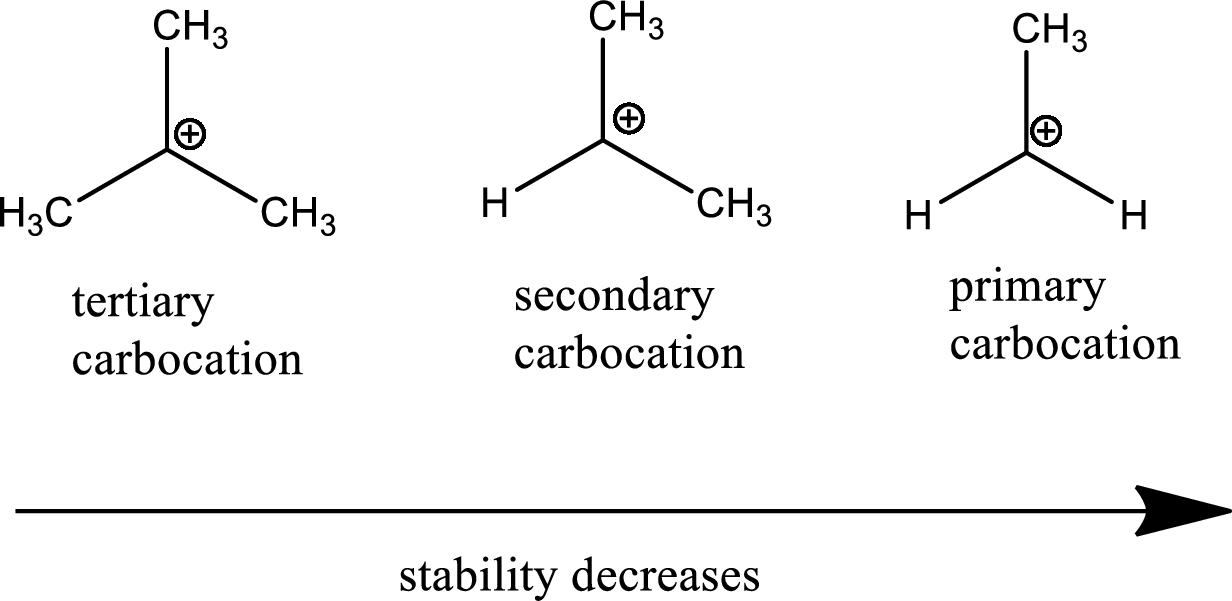
Nucleophile: donates pair of electrons to positively charged substrate resulting in the formation of chemical bond.
Electrophile: accepts pair of electrons from negatively charged substrate results in chemical bond formation.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Construct a molecular orbital energy-level diagram for BeH2. Sketch the MO pictures (schematic
representation) for the HOMO and LUMO of BeH2 [Orbital Potential Energies, H (1s): -13.6 eV; Be (2s):
-9.3 eV, Be (2p): -6.0 eV]
Indicate the isomers of the A(H2O)6Cl3 complex. State the type of isomerism they exhibit and explain it briefly.
State the formula of the compound potassium
μ-dihydroxydicobaltate (III) tetraoxalate.
Chapter 6 Solutions
Organic Chemistry
Ch. 6.2 - Using the BDE values from Appendix 3, calculate...Ch. 6.3 - Name and draw a structural formula for the product...Ch. 6.3 - Prob. 6.3PCh. 6.3 - Propose a mechanism for the addition of HI to...Ch. 6.3 - Prob. 6.5PCh. 6.3 - Propose a mechanism for the acid-catalyzed...Ch. 6.3 - The acid-catalyzed hydration of...Ch. 6.3 - Complete these reactions. (a) (b)Ch. 6.3 - Draw the structure of the chlorohydrin formed by...Ch. 6.4 - Draw structural formulas for the alkene that gives...
Ch. 6.5 - Prob. 6.11PCh. 6.5 - Prob. 6.12PCh. 6.5 - What alkene with the molecular formula C6H12, when...Ch. 6 - Prob. 6.15PCh. 6 - Prob. 6.16PCh. 6 - Predict the organic product(s) of the reaction of...Ch. 6 - Prob. 6.18PCh. 6 - Prob. 6.20PCh. 6 - Draw a structural formula for an alkene with the...Ch. 6 - Account for the fact that addition of HCl to...Ch. 6 - Account for the fact that treating propenoic acid...Ch. 6 - Draw a structural formula for the alkene with the...Ch. 6 - Draw the alternative chair conformations for the...Ch. 6 - Draw a structural formula for the cycloalkene with...Ch. 6 - Reaction of this bicycloalkene with bromine in...Ch. 6 - Terpin, prepared commercially by the...Ch. 6 - Propose a mechanism for this reaction and account...Ch. 6 - Treating 2-methylpropene with methanol in the...Ch. 6 - When 2-pentene is treated with Cl2 in methanol,...Ch. 6 - Treating cyclohexene with HBr in the presence of...Ch. 6 - Propose a mechanism for this reaction. 1-Pentane...Ch. 6 - Treating 4-penten-1-ol with bromine in water forms...Ch. 6 - Prob. 6.35PCh. 6 - Prob. 6.36PCh. 6 - Reaction of -pinene with borane followed by...Ch. 6 - Write structural formulas for the major organic...Ch. 6 - Draw the structural formula of the alkene that...Ch. 6 - Consider the following reaction. (a) Draw a...Ch. 6 - Prob. 6.42PCh. 6 - Prob. 6.43PCh. 6 - Show how to convert ethylene to these compounds....Ch. 6 - Show how to convert cyclopentene into these...Ch. 6 - Prob. 6.46PCh. 6 - Describe the stereochemistry of the bromohydrin...Ch. 6 - Prob. 6.49PCh. 6 - Treating 1,3-butadiene with 1 mole of HBr gives a...Ch. 6 - In this chapter, we studied the mechanism of the...Ch. 6 - As we have seen in this chapter, carbon-carbon...Ch. 6 - Prob. 6.53PCh. 6 - Prob. 6.54P
Knowledge Booster
Similar questions
- Consider the reaction of the cyclopentanone derivative shown below. i) NaOCH2CH3 CH3CH2OH, 25°C ii) CH3!arrow_forwardWhat constitutes a 'reference material', and why does its utilization play a critical role in the chemical analysis of food products? Provide examples.arrow_forwardExplain what calibration is and why it is essential in relation to food analysis. Provide examples.arrow_forward
- The cobalt mu-hydroxide complex cobaltate(III) of potassium is a dinuclear complex. Correct?arrow_forwardThe cobalt mi-hydroxide complex cobaltate(III) of potassium is a dinuclear complex. Correct?arrow_forward3. Arrange the different acids in Exercise B # 2 from the strongest (1) to the weakest acid (10). 1. 2. (strongest) 3. 4. 5. 6. 7. 8. 9. 10 10. (weakest)arrow_forward
- Name Section Score Date EXERCISE B pH, pOH, pка, AND PKD CALCULATIONS 1. Complete the following table. Solution [H+] [OH-] PH РОН Nature of Solution A 2 x 10-8 M B 1 x 10-7 M C D 12.3 6.8 2. The following table contains the names, formulas, ka or pka for some common acids. Fill in the blanks in the table. (17 Points) Acid Name Formula Dissociation reaction Ka pka Phosphoric acid H₂PO₁ H3PO4 H++ H₂PO 7.08 x 10-3 Dihydrogen H₂PO H₂PO H+ HPO 6.31 x 10-6 phosphate Hydrogen HPO₁ 12.4 phosphate Carbonic acid H2CO3 Hydrogen HCO 6.35 10.3 carbonate or bicarbonate Acetic acid CH,COOH 4.76 Lactic acid CH₂CHOH- COOH 1.38 x 10 Ammonium NH 5.63 x 10-10 Phenol CH₂OH 1 x 10-10 Protonated form CH3NH3* 3.16 x 10-11 of methylaminearrow_forwardIndicate whether it is true that Co(III) complexes are very stable.arrow_forwardMnO2 acts as an oxidant in the chlorine synthesis reaction.arrow_forward
- In Potassium mu-dihydroxydicobaltate (III) tetraoxalate K4[Co2(C2O4)4(OH)2], indicate whether the OH ligand type is bidentate.arrow_forwardImagine an electrochemical cell based on these two half reactions with electrolyte concentrations as given below: Oxidation: Pb(s) → Pb2+(aq, 0.10 M) + 2 e– Reduction: MnO4–(aq, 1.50 M) + 4 H+(aq, 2.0 M) + 3 e– → MnO2(s) + 2 H2O(l) Calculate Ecell (assuming temperature is standard 25 °C).arrow_forward: ☐ + Draw the Fischer projection of the most common naturally-occurring form of aspartate, with the acid group at the top and the side chain at the bottom. Important: be sure your structure shows the molecule as it would exist at physiological pH. Click and drag to start drawing a structure. ✓arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
