
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6, Problem 6.34P
Treating 4-penten-1-ol with bromine in water forms a cyclic bromoether.
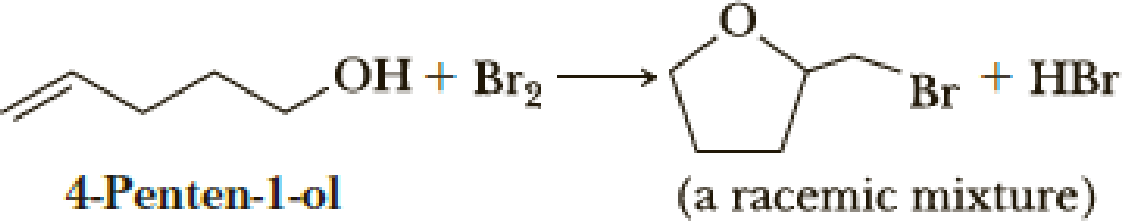
Account for the formation of this product rather than a bromohydrin as was formed in Problem 6.33
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Draw structures of the following compounds and identify their role:
mCPBA
(MCPBA)
DMS
Py
9-BBN
LAH
Sia₂BH
TsCI
PCC
t-BuOK
LDA
MeLi
n-BuLi
DMSO
DMF
Sodium Borohydride
Lithium DiisopropylAmide
2
Using Luther's rule, calculate the reference potential of the Hg2+/Hg redox electrode.
DATA: Electrode potentials E° = 0,854 V y E 0,788 V
Hg2+/Hg
2+
Hg2/Hg
1) NaNH2 (excess)
1) NaNH2
CI CI
2) H₂O
2) Mel
1) 03
2) (CH3)2S
Na
NH3 (liquid)
1
Chapter 6 Solutions
Organic Chemistry
Ch. 6.2 - Using the BDE values from Appendix 3, calculate...Ch. 6.3 - Name and draw a structural formula for the product...Ch. 6.3 - Prob. 6.3PCh. 6.3 - Propose a mechanism for the addition of HI to...Ch. 6.3 - Prob. 6.5PCh. 6.3 - Propose a mechanism for the acid-catalyzed...Ch. 6.3 - The acid-catalyzed hydration of...Ch. 6.3 - Complete these reactions. (a) (b)Ch. 6.3 - Draw the structure of the chlorohydrin formed by...Ch. 6.4 - Draw structural formulas for the alkene that gives...
Ch. 6.5 - Prob. 6.11PCh. 6.5 - Prob. 6.12PCh. 6.5 - What alkene with the molecular formula C6H12, when...Ch. 6 - Prob. 6.15PCh. 6 - Prob. 6.16PCh. 6 - Predict the organic product(s) of the reaction of...Ch. 6 - Prob. 6.18PCh. 6 - Prob. 6.20PCh. 6 - Draw a structural formula for an alkene with the...Ch. 6 - Account for the fact that addition of HCl to...Ch. 6 - Account for the fact that treating propenoic acid...Ch. 6 - Draw a structural formula for the alkene with the...Ch. 6 - Draw the alternative chair conformations for the...Ch. 6 - Draw a structural formula for the cycloalkene with...Ch. 6 - Reaction of this bicycloalkene with bromine in...Ch. 6 - Terpin, prepared commercially by the...Ch. 6 - Propose a mechanism for this reaction and account...Ch. 6 - Treating 2-methylpropene with methanol in the...Ch. 6 - When 2-pentene is treated with Cl2 in methanol,...Ch. 6 - Treating cyclohexene with HBr in the presence of...Ch. 6 - Propose a mechanism for this reaction. 1-Pentane...Ch. 6 - Treating 4-penten-1-ol with bromine in water forms...Ch. 6 - Prob. 6.35PCh. 6 - Prob. 6.36PCh. 6 - Reaction of -pinene with borane followed by...Ch. 6 - Write structural formulas for the major organic...Ch. 6 - Draw the structural formula of the alkene that...Ch. 6 - Consider the following reaction. (a) Draw a...Ch. 6 - Prob. 6.42PCh. 6 - Prob. 6.43PCh. 6 - Show how to convert ethylene to these compounds....Ch. 6 - Show how to convert cyclopentene into these...Ch. 6 - Prob. 6.46PCh. 6 - Describe the stereochemistry of the bromohydrin...Ch. 6 - Prob. 6.49PCh. 6 - Treating 1,3-butadiene with 1 mole of HBr gives a...Ch. 6 - In this chapter, we studied the mechanism of the...Ch. 6 - As we have seen in this chapter, carbon-carbon...Ch. 6 - Prob. 6.53PCh. 6 - Prob. 6.54P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- CI 1) n-BuLi 2) 1) 03 HH T&Cl 2) H₂O 2arrow_forwardHelp with a!arrow_forwardFor the following compound: HO -H Draw a mechanism for the tautomerization process under BASIC conditions: Mechanism A: H-O: H-OH H-O HH H-OO Mechanism B: H-Q Mechanism C: Θ OH H-O: Mechanism D: H-O H- H-OO C H-OO H- H- H-OO HH OH -H - HON H :OH H-Harrow_forward
- identify the product (or multiple products) for each of the following reactions: CI 1) NaNH2 (excess) ठ Cl 2) H₂O Hz H₂SO₂, H₂O HgSO Lindlar's catalyst 1) n-BuLi 2) 1)9-BBN 2) H₂O, NaOH ? Br H A B C afó gó H OA B O c OD E OF D E F H H Na, NHarrow_forwardIdentify the product (or multiple products) for each of the following reactions: ? or CI CI 1) NaNHz (excess) 2) H₂O OA OB O C OD OE OF H₂SO₂, H₂O Hq50. 1) n-BuLi 2) Br 1) 9-BBN 2) H₂O₂, NaOH A B H H متته D E H H H H C H H F H H H₂ Lindlar's catalyst Na NHarrow_forwardIdentify the product (or multiple products) for each of the following reactions: O A OB Oc OD OE OF CI CI 1) NaNH2 (excess) 2) H₂O H₂ H₂SO2, H₂O HgSO Lindlar's catalyst 1) n-BuLi 2) Br 1)9-BBN 2) H₂O₂, NaOH ? Na, NH3 C H A H H مننه مننه منن مننه H F H H E مند H D H Harrow_forward
- For the following compound: HO H Draw a mechanism for the tautomerization process under BASIC conditions: Mechanism A: + H-O: H-OH₂ H Mechanism B: H-Ö: HO-H H-OO -H H HH H H HH H-O: H-OO H-OO -H H e -H : OH Θ Mechanism C: Θ A : OH H-O: H H H-O-H 0. Mechanism D: e.. : OH :0 H H-O-H H-O: H-OO :O H -H H H сём H 0 :0 + H Θ H H H-arrow_forwardFor the following compound: H OH Draw a mechanism for the tautomerization process under ACIDIC conditions: Mechanism A: Θ :OH O O-H HO 0: Mechanism B: :O-H e.. Θ :OH Mechanism C: H HO-H :0: Θ 0: H H e.. : OH 0: "Θ HH O. :OH :OH O-H O-H Mechanism D: :OH H-OH₂ :OH HO-H 0: © O-H H HH 0: HHarrow_forwardHelp w c!arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License