
(a) Interpretation:
It is to be determined whether the given reactant is suitable for a reaction involving ethanol as a solvent with respect to leveling effect.
Concept introduction:
The solvent affects the properties of bases and acids. This effect is referred as leveling effect. For an acid-base reaction, the basicity of the solvent levels or limits the strength of the strong acid. Similarly, the acidity of the solvent levels the strength of the strong base. With respect to the leveling effect, a solvent is unsuitable for a particular reactant R if R is a stronger acid that the solvent’s conjugate acid (i.e., R has the lower pKa) or if R is a stronger base than the solvent’s conjugate base (i.e., the conjugate acid of R has a higher pKa than the solvent).
Answer to Problem 6.45P
With respect to the leveling effect, ethanol is not a suitable solvent for a reaction involving
Explanation of Solution
The reaction of
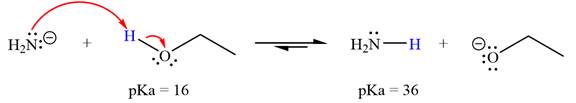
Ethanol,
The solvent effect on the reactant is determined with respect to the leveling effect.
(b)
Interpretation:
It is to be determined whether the given reactant is suitable for a reaction involving ethanol as a solvent with respect to leveling effect.
Concept introduction:
The solvent affects the properties of bases and acids. This effect is referred as leveling effect. For an acid-base reaction, the basicity of the solvent levels or limits the strength of the strong acid. Similarly, the acidity of the solvent levels the strength of the strong base. With respect to the leveling effect, a solvent is unsuitable for a particular reactant R if R is a stronger acid that the solvent’s conjugate acid (i.e., R has the lower pKa) or if R is a stronger base than the solvent’s conjugate base (i.e., the conjugate acid of R has a higher pKa than the solvent).
Answer to Problem 6.45P
With respect to the leveling effect, ethanol is a suitable solvent for a reaction involving
Explanation of Solution
The reaction of the acetate ion
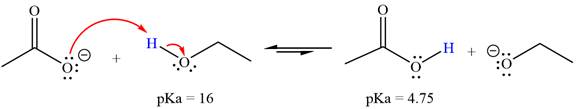
Acetic acid,
The solvent effect on the reactant is determined with respect to the leveling effect.
(c)
Interpretation:
It is to be determined whether the given reactant is suitable for a reaction involving ethanol as a solvent with respect to leveling effect.
Concept introduction:
The solvent affects the properties of bases and acids. This effect is referred as leveling effect. For an acid-base reaction, the basicity of the solvent levels or limits the strength of the strong acid. Similarly, the acidity of the solvent levels the strength of the strong base. With respect to the leveling effect, a solvent is unsuitable for a particular reactant R if R is a stronger acid that the solvent’s conjugate acid (i.e., R has the lower pKa) or if R is a stronger base than the solvent’s conjugate base (i.e., the conjugate acid of R has a higher pKa than the solvent).
Answer to Problem 6.45P
With respect to the leveling effect, ethanol is a suitable solvent for a reaction involving chloride ion
Explanation of Solution
The reaction of chloride ion
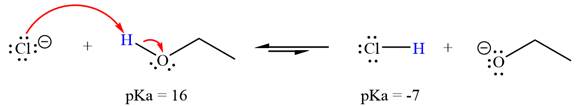
Hydrochloric acid,
The solvent effect on the reactant is determined with respect to the leveling effect.
(d)
Interpretation:
It is to be determined whether the given reactant is suitable for a reaction involving ethanol as a solvent with respect to leveling effect.
Concept introduction:
The solvent affects the properties of bases and acids. This effect is referred as leveling effect. For an acid-base reaction, the basicity of the solvent levels or limits the strength of the strong acid. Similarly, the acidity of the solvent levels the strength of the strong base. With respect to the leveling effect, a solvent is unsuitable for a particular reactant R if R is a stronger acid that the solvent’s conjugate acid (i.e., R has the lower pKa) or if R is a stronger base than the solvent’s conjugate base (i.e., the conjugate acid of R has a higher pKa than the solvent).
Answer to Problem 6.45P
With respect to the leveling effect, ethanol is a suitable solvent for a reaction involving phenoxide ion (
Explanation of Solution
The reaction of phenoxide ion (
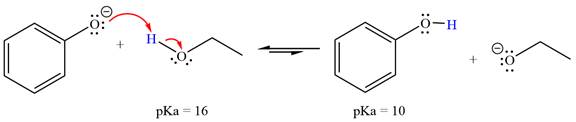
Phenol,
The solvent effect on the reactant is determined with respect to the leveling effect.
(e)
Interpretation:
It is to be determined whether the given reactant is suitable for a reaction involving ethanol as a solvent with respect to leveling effect.
Concept introduction:
The solvent affects the properties of bases and acids. This effect is referred as leveling effect. For an acid-base reaction, the basicity of the solvent levels or limits the strength of the strong acid. Similarly, the acidity of the solvent levels the strength of the strong base. With respect to the leveling effect, a solvent is unsuitable for a particular reactant R if R is a stronger acid that the solvent’s conjugate acid (i.e., R has the lower pKa) or if R is a stronger base than the solvent’s conjugate base (i.e., the conjugate acid of R has a higher pKa than the solvent).
Answer to Problem 6.45P
With respect to the leveling effect, ethanol is a suitable solvent for a reaction involving
Explanation of Solution
The reaction of cyanide ion
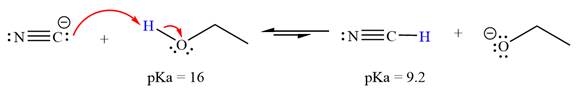
Acetylene,
The solvent effect on the reactant is determined with respect to the leveling effect.
(f)
Interpretation:
It is to be determined whether the given reactant is suitable for a reaction involving ethanol as a solvent with respect to leveling effect.
Concept introduction:
The solvent affects the properties of bases and acids. This effect is referred as leveling effect. For an acid-base reaction, the basicity of the solvent levels or limits the strength of the strong acid. Similarly, the acidity of the solvent levels the strength of the strong base. With respect to the leveling effect, a solvent is unsuitable for a particular reactant R if R is a stronger acid that the solvent’s conjugate acid (i.e., R has the lower pKa) or if R is a stronger base than the solvent’s conjugate base (i.e., the conjugate acid of R has a higher pKa than the solvent).
Answer to Problem 6.45P
With respect to the leveling effect, ethanol is not a suitable solvent for a reaction involving
Explanation of Solution
The reaction of propyl group
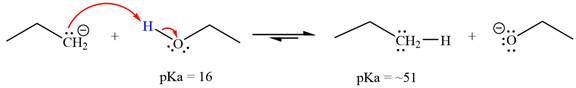
Ethanol,
The solvent effect on the reactant is determined with respect to the leveling effect.
Want to see more full solutions like this?
Chapter 6 Solutions
EBK ORGANIC CHEMISTRY: PRINCIPLES AND M
- Which of the following dienophiles is most reactive in a Diels-Alder reaction: Please explain why the correct answer to this question is option 5. Please provide a detailed explanation.arrow_forwardWhich of the following would you expect to be aromatic? Please provide a detailed explanation.arrow_forwardDraw the enantiomer and diastereomers of the following molecule. Label each type of stereoisomers. Label each chiral center as R or S. HOarrow_forward
- Which diene and dienophile would you choose to synthesize the following compound? Please provide a detailed explanation. Please include a drawing showing the mechanism of the synthesis. Please also explain why it is the correct diene and dienophile.arrow_forwardUsing the sketcher below, draw the structure of N-ethyldecylamine. Answer: 0 ୨୫) . 始 {n [ ]t ?arrow_forwardWhich of the following would you expect to be aromatic? Please provide a detailed explanation.arrow_forward
- Identify the characteristic signals that you would expect in the diagnostic region of an IR spectrum of each of the following compounds. a. H₂N b.arrow_forwardWhat is the lowest energy chair for the following cyclohexane? ' || || a. b. " " d.arrow_forwardAnswer the following questions using the below figure: Potential Energy ри Reaction Progress a. How many transition states occur in this reaction? b. How many intermediates occur in this reaction? c. Is this reaction spontaneous or nonspontaneous? d. Does this reaction have a positive or negative AG? e. Label the activation energy(ies).arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

