
Nitric acid is a chemical intermediate primarily used in the synthesis of ammonium nitrate, which is used in the manufacture of fertilizers. The acid also is important in the production of other nitrates and in the separation of metals from ores.
Nitric acid may be produced by oxidizing ammonia to nitric oxide over a platinum—rhodium catalyst, then oxidizing the nitric oxide to nitrogen dioxide in a separate unit where it is absorbed in water to form an aqueous solution of nitric acid.
The reaction sequence is as follows:
where, unless otherwise speci?ed, the species are gases. A side reaction in which ammonia is oxidized to form nitrogen and water can lower product yield:
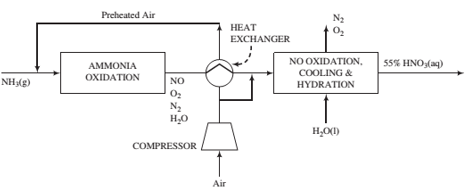
Ammonia vapor produced by vaporizng pure liquid ammonia at 820 kPa absolute is mixed with air, and the combined stream enters the ammonia oxidation unit. Air at 30°C, 1 atm absolute, and 50% relative humidity is compressed and fed to the process. A fraction of the air is sent to the cooling and hydration units, while the remainder is passed through a heat exchanger and mixed with the ammonia. The total oxygen fed to the process is the amount stoichiometrically required to convert all of the ammonia to HNO3, while the fraction sent to the ammonia oxidizer corresponds to the stoichiometric amount required to convert ammonia to NO.
The ammonia reacts completely in the oxidizer, with 97% forming NO and the rest forming N2. Only a negligible amount of NO2is formed in the oxidizer. However, the gas leaving the oxidizer is subjected to a series of cooling and hydration steps in which the NO is completely oxidized to NO2, which in turn combines with water (some of which is present in the gas from the oxidizer and the rest is added) to form a 55 wt% aqueous solution of nitric acid. The product gas from the process may be takento contain only N2and O2.
(a) Taking a basis of 100 kmol of ammonia fed to the process, calculate (i) the volumes (m3) of the ammonia vapor and air fed to the process using the compressibility-factor equation of state; (ii) the amount (kmol) and composition (in mole fractions) of the gas leaving the oxidation unit; (m) the required volume of liquid water(m3) that must be fed to the cooling and hydration units; and (iv) the fraction of the air fed to the ammonia oxidizer.
(b) Scale the results from Part (a) to a new basis of 100 metric tons per hour of 55% nitiic acid solution.
Exploratory Exercises—Research and Discover (c) Nitrogen oxides (collectively referred to as NOx) are a category of pollutants that are formed in many ways, including processes like that described in this problem. List the annual emission rates of the three largest sources of NOxemissions in your home region. What are the effects of exposure to excessive concentrations of NOx?
(d) A platinum—rhodium catalyst is used in ammonia oxidation. Explain the function of the catalyst, describe its structure, and explain the relationship of the structure to the function.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Elementary Principles Of Chemical Processes
Additional Engineering Textbook Solutions
Degarmo's Materials And Processes In Manufacturing
Starting Out with Java: From Control Structures through Data Structures (4th Edition) (What's New in Computer Science)
Starting Out with Python (4th Edition)
Modern Database Management
Database Concepts (8th Edition)
Java: An Introduction to Problem Solving and Programming (8th Edition)
- Problem 9.13 A 70 mm long line PQ does not have H.T. and V.T. One end of the line is 30 mm in front of the V.P. and 20 mm above the H.P. Draw its projections. Interpretation As the line PQ does not have H.T. and V.T., it is parallel to both H.P. and V.P. Construction Refer to Fig. 9.13. 1. Draw a reference line xy. Mark point p' 20 mm above xy and point p 30 mm below xy. 2. Draw a 70 mm long line p'a' parallel to xy to repre- sent the front view. X 20 p Fig. 9.13 3. Also, draw a 70 mm long line pq parallel to xy to represent the top view. 70 q yarrow_forwardProblem 10.19 A square lamina ABCD of side 40 mm is suspended from a point O such that its surface is inclined at 30° to the V.P. The point O lies on the side AB 12 mm away from A. Draw its projections. Construction Refer to Fig. 10.19. 1. First stage Draw a square a'b'c'd keeping a'd' parallel to xy. Mark a point o' on a'ď at a distance 12 mm from end a' as the point of suspension. Also, mark the centre of the square g' to represent the centre of gravity. 2. Second stage Reproduce the front view of first stage such that o'g' is perpendicular to xy. Project corners and obtain bd as the top view. 3. Third stage Reproduce the top view keeping bd inclined at 30° to xy. Obtain new points a', b', c' and ď' in the front view by joining the points of intersection of the vertical projectors drawn from points a, b, c and d of the third stage with the corresponding horizontal locus lines drawn from points a', b', c' and ď of the second stage. Join new a'b'c'd to represent the final front view.arrow_forwardProblem 10.15 A circular plane of diameter 50 mm is resting on a point of the circumference on the V.P. The plane is inclined at 30° to the V.P. and the centre is 35 mm above the H.P. Draw its projections.arrow_forward
- You are asked to manufacture 10 kg of polyester with a number-average molecular weight of 1000 by polymerizing butane-1,4-diol(HO(CH2)4OH) with adipic acid (HOOC-(CH2)4-COOH).a) What weight of diol and diacid do you need, respectively? To whatextent, p, should the reaction be carried out to? Assume a stoichiometricbalance.b) What are the number and weight fractions of dimer, trimer and tetramerat this point in the reaction?c) Because of the polymerization by dehydration to olefin, 3 mol% of thediol will be lost. What would be the number-average molecular weightwhen the reaction is carried out to the same extent? How could you offsetthis loss so that the desired molecular weightarrow_forward9.4. A PID temperature controller is at steady state with an output pressure of 9 psig. The set point and process temperature are initially the same. At time = 0, the set point is increased at the rate of 0.5°F/min. The motion of the set point is in the direction of lower temperatures. If the current settings are PART 3 LINEAR CLOSED-LOOP SYSTEMS Ke = 2 psig/°F Ti = 1.25 min TD = 0.4 min plot the output pressure versus time.arrow_forward9.6. A PI controller has the transfer function Determine the values of K, and T. 5s + 10 Ge Sarrow_forward
- 9.5. The input & to a PI controller is shown in Fig. P9-5. Plot the output of the controller if Ke 2 and 0.50 min. - E 0.5 0 -0.5 0 FIGURE P9-5 2 4 t, minarrow_forward9.3. An ideal PD controller has the transfer function P Ke (TDs + 1) E An actual PD controller had the transfer function P = Ke E TDS +1 (TDIẞ)s +1 where ẞis a large constant in an industrial controller. If a unit-step change in error is introduced into a controller having the second transfer function, show that P(1) = Kc (1 + Ae˜¯BD) where A is a function of ẞwhich you are to determine. For ẞ=5 and K = 0.5, plot P(t) versus tl tp. As ẞ, show that the unit-step response approaches that for the ideal controller.arrow_forward9.1. A pneumatic PI temperature controller has an output pressure of 10 psig when the set point and process temperature coincide. The set point is suddenly increased by 10°F (i.e., a step change in error is introduced), and the following data are obtained: Time, s psig 0- 10 0+ 8 20 7 60 90 5 3.5 Determine the actual gain (psig per degree Fahrenheit) and the integral time.arrow_forward
- 2. A unit-step change in error is introduced into a PID controller. If Ke TD = 0.5, plot the response of the controller P(t). = =10, 1, andarrow_forwardA distribution of values is normal with a mean of 211 and a standard deviation of 50.4. Find the probability that a randomly selected value is between 59.8 and 155.6. P(59.8 X 155.6) = Enter your answer as a number accurate to 4 decimal places. Answers obtained using exact z-scores or z- scores rounded to 3 decimal places are accepted.arrow_forwardTopic: Production of propylene glycol from glycerol derived from palm oil. QUESTION:Estimate capital items, operating costs and economics of the plant. Finally, report the estimatedreturn.The Detailed Factorial Method with approximately 25% accuracy must be used for detailedeconomic evaluation.Plant lifetime is fixed at 15 years.1) Depreciation and TaxesCalculate the depreciation using a suitable method (e.g., straight-line, declining balance) andincorporate tax implications based on current tax laws applicable to chemical plants. Use following attached Process Flow Diagram as reference for this question.arrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





