
Dehydration of natural gas is necessary to prevent the formation of gas hydrates, which can plug valves and other components of a gas pipeline, and also to reduce potential corrosion problems. Water removal can be accomplished as shown in the following schematic diagram:
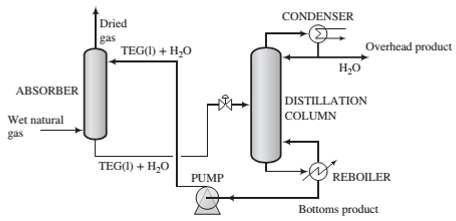
Natural gas containing
(a) Draw and completely label a ?owchart of this process. Calculate the mass ?ow rate (lbm/day) and volumetric ?ow rate (ft3/day) of the overhead product from the distillation column.
(b) The greatest possible amount of dehydration is achieved if the gas leaving the absorption column is in equilibrium with the solvent entering the column. If the Henry's law constant for water in TEG at 90°F is 0.398 psia/mol fraction, what is the maximum allowable mole fraction of water in the solvent fed to the absorber‘?
(c) A column of in?nite height would be required to achieve equilibrium between the gas and liquid at the top of the absorber. For the desired separation to be achieved in practice, the mole fraction of water in the entering solvent must be less than the value calculated in Part (b). Suppose it is 80% of that value and the ?ow rate of TEG in the recirculating solvent is 37 lbmTEG/lbmwater absorbed in the column. Calculate the ?ow rate (lbm/day) of the solvent stream entering the absorber and the mole fraction of water in the solvent stream leaving the absorber.
(d) What is the purpose of the distillation column in the process? (Hint: Think about how the process would operate without it.)
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Elementary Principles Of Chemical Processes
Additional Engineering Textbook Solutions
Electric Circuits. (11th Edition)
Starting Out with Java: From Control Structures through Data Structures (4th Edition) (What's New in Computer Science)
Management Information Systems: Managing The Digital Firm (16th Edition)
Database Concepts (8th Edition)
Computer Science: An Overview (13th Edition) (What's New in Computer Science)
- 9.3. An ideal PD controller has the transfer function P Ke (TDs + 1) E An actual PD controller had the transfer function P = Ke E TDS +1 (TDIẞ)s +1 where ẞis a large constant in an industrial controller. If a unit-step change in error is introduced into a controller having the second transfer function, show that P(1) = Kc (1 + Ae˜¯BD) where A is a function of ẞwhich you are to determine. For ẞ=5 and K = 0.5, plot P(t) versus tl tp. As ẞ, show that the unit-step response approaches that for the ideal controller.arrow_forward9.1. A pneumatic PI temperature controller has an output pressure of 10 psig when the set point and process temperature coincide. The set point is suddenly increased by 10°F (i.e., a step change in error is introduced), and the following data are obtained: Time, s psig 0- 10 0+ 8 20 7 60 90 5 3.5 Determine the actual gain (psig per degree Fahrenheit) and the integral time.arrow_forward2. A unit-step change in error is introduced into a PID controller. If Ke TD = 0.5, plot the response of the controller P(t). = =10, 1, andarrow_forward
- A distribution of values is normal with a mean of 211 and a standard deviation of 50.4. Find the probability that a randomly selected value is between 59.8 and 155.6. P(59.8 X 155.6) = Enter your answer as a number accurate to 4 decimal places. Answers obtained using exact z-scores or z- scores rounded to 3 decimal places are accepted.arrow_forwardTopic: Production of propylene glycol from glycerol derived from palm oil. QUESTION:Estimate capital items, operating costs and economics of the plant. Finally, report the estimatedreturn.The Detailed Factorial Method with approximately 25% accuracy must be used for detailedeconomic evaluation.Plant lifetime is fixed at 15 years.1) Depreciation and TaxesCalculate the depreciation using a suitable method (e.g., straight-line, declining balance) andincorporate tax implications based on current tax laws applicable to chemical plants. Use following attached Process Flow Diagram as reference for this question.arrow_forwardTopic: Production of propylene glycol from glycerol derived from palm oil. QUESTION:Estimate capital items, operating costs and economics of the plant. Finally, report the estimatedreturn.The Detailed Factorial Method with approximately 25% accuracy must be used for detailedeconomic evaluation.Plant lifetime is fixed at 15 years.1) Revenue EstimationEstimate the annual revenue based on the production capacity, product selling price, and marketdemand. Groups should also consider potential market fluctuations. Use following attached Process Flow Diagram as reference for this question.arrow_forward
- Topic: Production of propylene glycol from glycerol derived from palm oil. QUESTION:Estimate capital items, operating costs and economics of the plant. Finally, report the estimatedreturn.The Detailed Factorial Method with approximately 25% accuracy must be used for detailedeconomic evaluation.Plant lifetime is fixed at 15 years.TASKS:1) Capital Cost EstimationProvide a detailed breakdown of the initial capital investment, including land, building,equipment, and installation costs. Include any assumptions made in the estimation. Use following attached Process Flow Diagram as reference for this question.arrow_forwardTopic: Production of propylene glycol from glycerol derived from palm oil. QUESTION:Estimate capital items, operating costs and economics of the plant. Finally, report the estimatedreturn.The Detailed Factorial Method with approximately 25% accuracy must be used for detailedeconomic evaluation.Plant lifetime is fixed at 15 years.1) Breakeven Year CalculationUsing the cash flow analysis, calculate the breakeven year when the cumulative cash inflowequals the initial investment. Groups should graphically represent the breakeven point. Use following attached Process Flow Diagram as reference for this question.arrow_forwardTopic: Production of propylene glycol from glycerol derived from palm oil. QUESTION:Estimate capital items, operating costs and economics of the plant. Finally, report the estimatedreturn.The Detailed Factorial Method with approximately 25% accuracy must be used for detailedeconomic evaluation.Plant lifetime is fixed at 15 years.1) Cash Flow AnalysisDevelop a projected cash flow statement for the first 10 years of plant operation, consideringall the costs and revenues. Include working capital, loans, and interest payments if applicable. Use following attached Process Flow Diagram as reference for this question.arrow_forward
- Topic: Production of propylene glycol from glycerol derived from palm oil.QUESTION:Estimate capital items, operating costs and economics of the plant. Finally, report the estimatedreturn.The Detailed Factorial Method with approximately 25% accuracy must be used for detailedeconomic evaluation.Plant lifetime is fixed at 15 years.1) Operational Cost AnalysisCalculate the yearly operational costs, including raw materials, labor, utilities, maintenance,and other recurring expenses. Provide a clear explanation of how these costs are derived. Use following attached Process Flow Diagram as reference for this question.arrow_forwardChemical Engineering Questionarrow_forwardA steam boiler or steam generator is a device used to produce steam by transferring heat to water. In our case, the combustion chamber is fueled with propane (C3H8) at a flowrate of 50.0 mol/h in an excess air of 50%. Assume that both propane and air are fed at 25ºC and the combustion gases leave the chamber at 200ºC. Pressure can be assumed to be atmospheric.* Determine: 1. The heat obtained assuming complete combustion. Compare the results using elements or compounds 2. The steam flowrate that could be generated if the heat is directed to obtain superheated steam at 2 bar and 160ºC from saturated liquid water at this pressure solvearrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





