
A fuel cell is an electrochemical device in which hydrogen reacts with oxygen to produce water and DC electricity. A 1-watt proton-exchange membrane fuel cell (PEMFC) could be used for portable applications such as cellular telephones, and a 100-kW PEMFC could be used to power an automobile
The following reactions occur inside the PEMFC:
Anode:
Cathode:
Overall:
A ?owchart of a single cell of a PEMFC is shown below. The complete cell would consist of a stack of such cells in series, such as the one shown in Problem 9.19.
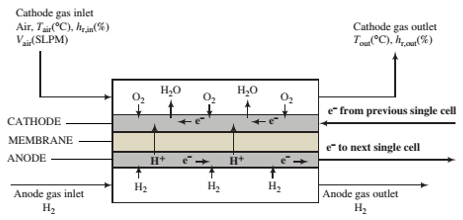
The cell consists of two gas channels separated by a membrane sandwiched between two ?at carbon-paper electrodes—the anode and the cathode—that contain imbedded platinum particles. Hydrogen ?ows into the anode chamber and contacts the anode, where H2molecules are catalyzed by the platinum to dissociate and ionize to form hydrogen ions (protons) and electrons. The electrons are conducted through the carbon ?bers of the anode to an external circuit, where they pass to the cathode of the next cell in the stack. The hydrogen ions permeate from the anode through the membrane to the cathode.
Humid air is fed into the cathode chamber, and at the cathode O2molecules are catalytically split to form oxygen atoms, which combine with the hydrogen ions coming through the membrane and electrons coming from the external circuit to form water. The water desorbs into the cathode gas and is carried out of the cell. The membrane material is a hydrophilic polymer that absorbs water molecules and facilitates the transport of the hydrogen ions from the anode to the cathode. Electrons come from the anode of the cell at one end of the stack and ?ow through an external circuit to drive the device that the fuel cell is powering, while the electrons coming from the device ?ow back to the cathode at the opposite end of the stack to complete the circuit.
It is important to keep the water content of the cathode gas between upper and lower limits. lf the content reaches a value for which the relative humidity would exceed 100%, condensation occurs at the cathode (?ooding), and the entering oxygen must diffuse through a liquid water ?lm before it cart react. The rate of this diffusion is much lower than the rate of diffusion through the gas film normally adjacent to the cathode, and so the performance of the fuel cell deteriorates. On the other hand, if there is not enough water in the cathode gas (less than 85% relative humidity), the membrane dries out and cannot transport hydrogen efficiently, which also leads to reduced performance.
A 400-cell 300—volt PEMFC operates at steady state with a power output of 36 kW. The air fed to the cathode side is at 200°C and roughly 1.0 atm (absolute) with a relative humidity of 70.0% and a volumetric ?ow rate of
(a) Explain in your own words what happens in a single cell of a PEMFC.
(b) The stoichiometric hydrogen requirement for a PEMFC is given by
(c) Use the expression of Part (b) to determine the molar rates of oxygen consumed and water generated in the unit with the given speci?cations, both in units of mol/min. (Remember that power = voltage × current.) Then determine the relative humidity of the cathode exit stream,
(d) Determine the minimum cathode inlet ?ow rate in SLPM to prevent the fuel cell from ?ooding
Learn your wayIncludes step-by-step video

Chapter 6 Solutions
Elementary Principles Of Chemical Processes
Additional Engineering Textbook Solutions
Modern Database Management
Starting Out With Visual Basic (8th Edition)
Starting Out with C++ from Control Structures to Objects (9th Edition)
Starting Out with Java: From Control Structures through Objects (7th Edition) (What's New in Computer Science)
Starting Out with Java: From Control Structures through Data Structures (4th Edition) (What's New in Computer Science)
Introduction To Programming Using Visual Basic (11th Edition)
- Curved arrows are used to illustrate the flow of electrons. Follow the curved arrows and draw the structure of the missing reactants, intermediates, or products in the following mechanism. Include all lone pairs. Ignore stereochemistry. Ignore inorganic byproducts. H Br2 (1 equiv) H- Select to Draw Starting Alkene Draw Major Product I I H2O 四: ⑦.. Q Draw Major Charged Intermediate Iarrow_forwardNH (aq)+CNO (aq) → CO(NH2)2(s) Experiment [NH4] (M) [CNO] (M) Initial rate (M/s) 1 0.014 0.02 0.002 23 0.028 0.02 0.008 0.014 0.01 0.001 Calculate the rate contant for this reaction using the data provided in the table.arrow_forward2CIO2 + 20H-1 CIO31 + CIO2 + H2O Experiment [CIO2], M [OH-1], M 1 0.0500 0.100 23 2 0.100 0.100 3 0.100 0.0500 Initial Rate, M/s 0.0575 0.230 0.115 ... Given this date, calculate the overall order of this reaction.arrow_forward
- 2 3 .(be)_[Ɔ+(be)_OI ← (b²)_IƆO+ (be)_I Experiment [1-] M 0.005 [OCI-] 0.005 Initial Rate M/min 0.000275 0.0025 0.005 0.000138 0.0025 0.0025 0.000069 4 0.0025 0.0025 0.000140 Calculate the rate constant of this reaction using the table data.arrow_forward1 2 3 4 I(aq) +OCl(aq) → IO¯¯(aq) + Cl¯(aq) Experiment [I-] M 0.005 [OCI-] 0.005 Initial Rate M/min 0.000275 0.0025 0.005 0.000138 0.0025 0.0025 Calculate the overall order of this reaction using the table data. 0.0025 0.000069 0.0025 0.000140arrow_forwardH2O2(aq) +3 I¯(aq) +2 H+(aq) → 13(aq) +2 H₂O(l)· ••• Experiment [H2 O2]o (M) [I]o (M) [H+]。 (M) Initial rate (M/s) 1 0.15 0.15 0.05 0.00012 234 0.15 0.3 0.05 0.00024 0.3 0.15 0.05 0.00024 0.15 0.15 0.1 0.00048 Calculate the overall order of this reaction using the table data.arrow_forward
- The U. S. Environmental Protection Agency (EPA) sets limits on healthful levels of air pollutants. The maximum level that the EPA considers safe for lead air pollution is 1.5 μg/m³ Part A If your lungs were filled with air containing this level of lead, how many lead atoms would be in your lungs? (Assume a total lung volume of 5.40 L.) ΜΕ ΑΣΦ = 2.35 1013 ? atoms ! Check your rounding. Your final answer should be rounded to 2 significant figures in the last step. No credit lost. Try again.arrow_forwardY= - 0.039 (14.01) + 0.7949arrow_forwardSuppose 1.76 g of magnesium acetate (Mg (CH3CO2)2) are dissolved in 140. mL of water. Find the composition of the resulting electrolyte solution. In particular, list the chemical symbols (including any charge) of each dissolved ion in the table below. List only one ion per row. mEq Then, calculate the concentration of each ion in dwrite the concentration in the second column of each row. Be sure you round your answers to the L correct number of significant digits. ion Add Row mEq L x 5arrow_forward
- A pdf file of your hand drawn, stepwise mechanisms for the reactions. For each reaction in the assignment, you must write each mechanism three times (there are 10 reactions, so 30 mechanisms). (A) do the work on a tablet and save as a pdf., it is expected to write each mechanism out and NOT copy and paste the mechanism after writing it just once. Everything should be drawn out stepwise and every bond that is formed and broken in the process of the reaction, and is expected to see all relevant lone pair electrons and curved arrows.arrow_forwardNonearrow_forwardNonearrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





