
Concept explainers
(a)
Interpretation: The conformation which is present in higher concentration when
Concept introduction: The change in Gibbs free energy is represented by
If the
Answer to Problem 6.40P
The equatorial conformation is present in higher concentration in the given compound.
Explanation of Solution
Given
The value of
The equilibrium reaction of monosubstituted cyclohexane is shown below.
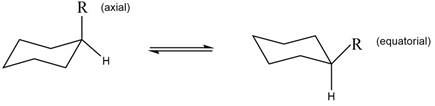
Figure 1
The value of
The equatorial conformation is present in higher concentration in the given compound.
(b)
Interpretation: The
Concept introduction: The change in Gibbs free energy is represented by
If the
Answer to Problem 6.40P
The
Explanation of Solution
The values of
The both given values are greater than
If the
Therefore, the
The
(c)
Interpretation: The
Concept introduction: The change in Gibbs free energy is represented by
If the
Answer to Problem 6.40P
The
Explanation of Solution
The values of
The equilibrium constant
Therefore, the
The
(d)
Interpretation: The
Concept introduction: The change in Gibbs free energy is represented by
If the
Answer to Problem 6.40P
The value of
Explanation of Solution
Given
The values of
The relationship between
As the value of
The value of
(e)
Interpretation: The explanation corresponding to the relation of size of
Concept introduction: The change in Gibbs free energy is represented by
If the
Answer to Problem 6.40P
The large size of
Explanation of Solution
The value
The large size of
Want to see more full solutions like this?
Chapter 6 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
- → Acetyl-CoA + 3NAD+ + 1FAD + 1ADP 2CO2 + CoA + 3NADH + 1FADH2 + 1ATP a. Which of the above are the reactants? b. Which of the above are the products? c. Which reactant is the electron donor? d. Which reactants are the electron acceptors? e. Which of the products are now reduced? f. Which product is now oxidized? g. Which process was used to produce the ATP? h. Where was the energy initially in this chemical reaction and where is it now that it is finished? i. Where was the carbon initially in this chemical reaction and where is it now that it is finished? j. Where were the electrons initially in this chemical reaction and where is it now that it is finished?arrow_forwardRank each of the following substituted benzene molecules in order of which will react fastest (1) to slowest (4) by electrophilic aromatic substitution. OCH 3 (Choose one) OH (Choose one) Br (Choose one) Explanation Check NO2 (Choose one) © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Aarrow_forwardFor each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Molecule Inductive Effects O donating O withdrawing O no inductive effects Resonance Effects Overall Electron-Density ○ donating ○ withdrawing O no resonance effects O electron-rich O electron-deficient O similar to benzene Cl O donating O withdrawing ○ donating ○ withdrawing O no inductive effects O no resonance effects O Explanation Check O electron-rich O electron-deficient similar to benzene X © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessarrow_forward
- Identifying electron-donating and For each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Molecule Inductive Effects NH2 ○ donating NO2 Explanation Check withdrawing no inductive effects Resonance Effects Overall Electron-Density ○ donating O withdrawing O no resonance effects O donating O withdrawing O donating withdrawing O no inductive effects Ono resonance effects O electron-rich electron-deficient O similar to benzene O electron-rich O electron-deficient O similar to benzene olo 18 Ar 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilityarrow_forwardRank each of the following substituted benzene molecules in order of which will react fastest (1) to slowest (4) by electrophilic aromatic substitution. Explanation Check Х (Choose one) OH (Choose one) OCH3 (Choose one) OH (Choose one) © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forwardAssign R or S to all the chiral centers in each compound drawn below porat bg 9 Br Brarrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

