
Concept explainers
(a)
Interpretation:
Missing curved arrows are to be supplied for the given proton transfer reaction. The relevant electrons are to be drawn if they are not shown.
Concept introduction:
In a proton transfer reaction, a proton is transferred from a Bronsted-Lowry acid to a Bronsted-Lowry base in a single elementary step in which one bond is broken and another is formed simultaneously. The curved arrow notation shows the movement of valence electrons, not atoms. The movement of two electrons is shown be using a double-barbed arrow. To represent bond breaking, the tail of the arrow originates from the center of a bond whereas to represent bond formation, the head of arrow points to an atom which forms the new bond, that is, σ bond or the region where the bond is formed if the new bond is a π bond.
Answer to Problem 6.39P
The missing curved arrow notation for the proton transfer reaction and relevant electrons is shown as
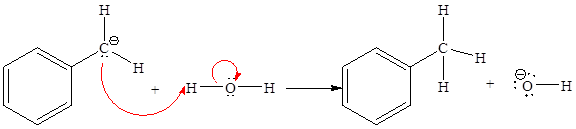
Explanation of Solution
The given proton transfer reaction is
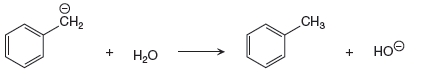
In the above reaction, the bond
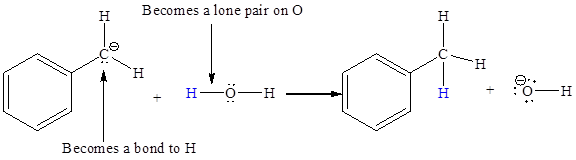
The appropriate movement of these valence electrons is shown by using curved arrow notations. One curved arrow is to be drawn from the lone pair on C to the H on water (highlighted blue) to illustrate the formation of
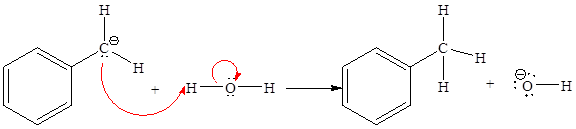
The curved arrow notation for the proton transfer of the given reaction is drawn on the basis of the movement of valence electrons involved in bond breaking and bond formation.
(b)
Interpretation:
Missing curved arrows are to be supplied for the given proton transfer reaction. The relevant electrons are to be drawn if they are not shown.
Concept introduction:
In a proton transfer reaction, a proton is transferred from a Bronsted-Lowry acid to a Bronsted-Lowry base in a single elementary step in which one bond is broken and another is formed simultaneously. The curved arrow notation shows the movement of valence electrons, not atoms. The movement of two electrons is shown be using a double-barbed arrow. To represent bond breaking, the tail of the arrow originates from the center of a bond whereas to represent bond formation, the head of arrow points to an atom which forms the new bond, that is, ![]() bond or the region where the bond is formed if the new bond is a
bond or the region where the bond is formed if the new bond is a ![]() bond.
bond.
Answer to Problem 6.39P
The missing curved arrow notation for the proton transfer reaction and relevant electrons is shown as
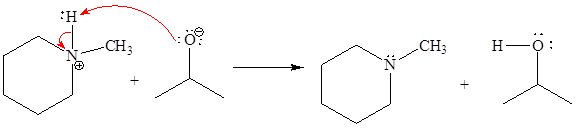
Explanation of Solution
The given proton transfer reaction is
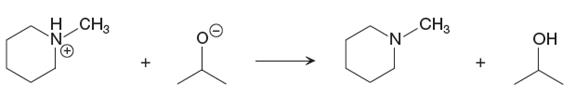
In the above reaction, the bond
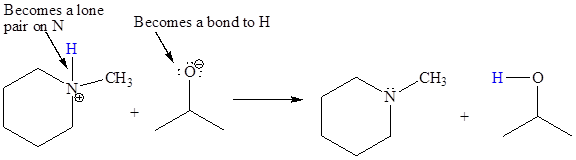
The appropriate movement of these valence electrons is shown by using curved arrow notations. One curved arrow is to be drawn from the lone pair on O to the H on N (highlighted blue) to illustrate the formation of the

The curved arrow notation for the proton transfer of the given reaction is drawn on the basis of the movement of valence electrons involved in bond breaking and bond formation.
(c)
Interpretation:
Missing curved arrows are to be supplied for the given proton transfer reaction. The relevant electrons are to be drawn if they are not shown.
Concept introduction:
In a proton transfer reaction, a proton is transferred from a Bronsted-Lowry acid to a Bronsted-Lowry base in a single elementary step in which one bond is broken and another is formed simultaneously. The curved arrow notation shows the movement of valence electrons, not atoms. The movement of two electrons is shown be using a double-barbed arrow. To represent bond breaking, the tail of the arrow originates from the center of a bond whereas to represent bond formation, the head of arrow points to an atom which forms the new bond, that is, ![]() bond or the region where the bond is formed if the new bond is a
bond or the region where the bond is formed if the new bond is a ![]() bond.
bond.
Answer to Problem 6.39P
The missing curved arrow notation for the proton transfer reaction and relevant electrons is shown as
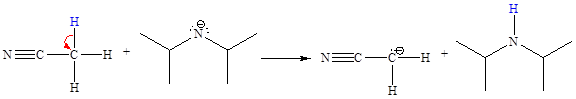
Explanation of Solution
The given proton transfer reaction is
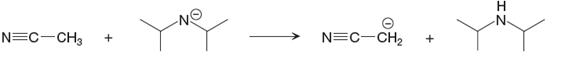
In the above reaction, the bond
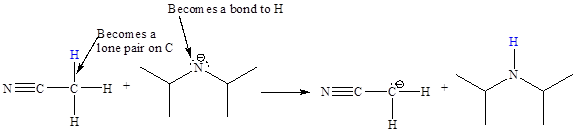
The appropriate movement of these valence electrons is shown by using curved arrow notations. One curved arrow is to be drawn from the lone pair on N to the H on C (highlighted blue) to illustrate the formation of the
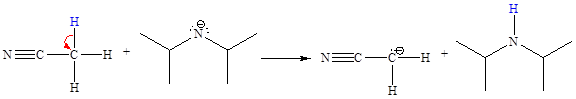
The curved arrow notation for the proton transfer of the given reaction is drawn on the basis of the movement of valence electrons involved in bond breaking and bond formation.
(d)
Interpretation:
Missing curved arrows are to be supplied for the given proton transfer reaction. The relevant electrons are to be drawn if they are not shown.
Concept introduction:
In a proton transfer reaction, a proton is transferred from a Bronsted-Lowry acid to a Bronsted-Lowry base in a single elementary step in which one bond is broken and another is formed simultaneously. The curved arrow notation shows the movement of valence electrons, not atoms. The movement of two electrons is shown be using a double-barbed arrow. To represent bond breaking, the tail of the arrow originates from the center of a bond whereas to represent bond formation, the head of arrow points to an atom which forms the new bond, that is, ![]() bond or the region where the bond is formed if the new bond is a
bond or the region where the bond is formed if the new bond is a ![]() bond.
bond.
Answer to Problem 6.39P
The missing curved arrow notation for the proton transfer reaction and relevant electrons is shown as
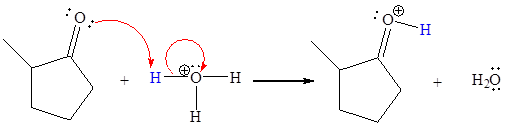
Explanation of Solution
The given proton transfer reaction is
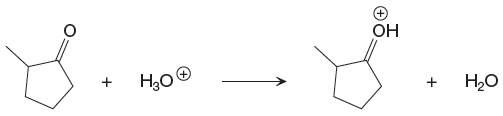
In the above reaction, the bond
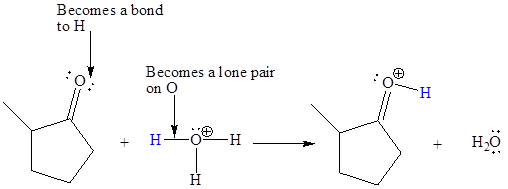
The appropriate movement of these valence electrons is shown by using curved arrow notations. One curved arrow is to be drawn from the lone pair on O to the H of
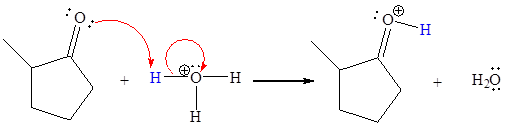
The curved arrow notation for the proton transfer of the given reaction is drawn on the basis of the movement of valence electrons involved in bond breaking and bond formation.
Want to see more full solutions like this?
Chapter 6 Solutions
Organic Chemistry: Principles And Mechanisms: Study Guide/solutions Manual (second)
- Q1: For each molecule, assign each stereocenter as R or S. Circle the meso compounds. Label each compound as chiral or achiral. OH HO CI Br H CI CI Br CI CI Xf x f g Br D OH Br Br H₂N R. IN Ill I -N S OMe D II H CO₂H 1/111 DuckDuckGarrow_forwardThese are synthesis questions. You need to show how the starting material can be converted into the product(s) shown. You may use any reactions we have learned. Show all the reagents you need. Show each molecule synthesized along the way and be sure to pay attention to the regiochemistry and stereochemistry preferences for each reaction. If a racemic molecule is made along the way, you need to draw both enantiomers and label the mixture as "racemic". All of the carbon atoms of the products must come from the starting material! ? H Harrow_forwardQ5: Draw every stereoisomer for 1-bromo-2-chloro-1,2-difluorocyclopentane. Clearly show stereochemistry by drawing the wedge-and-dashed bonds. Describe the relationship between each pair of the stereoisomers you have drawn.arrow_forward
- Classify each pair of molecules according to whether or not they can participate in hydrogen bonding with one another. Participate in hydrogen bonding CH3COCH3 and CH3COCH2CH3 H2O and (CH3CH2)2CO CH3COCH3 and CH₂ CHO Answer Bank Do not participate in hydrogen bonding CH3CH2OH and HCHO CH3COCH2CH3 and CH3OHarrow_forwardNonearrow_forwardQ4: Comparing (3S,4S)-3,4-dimethylhexane and (3R,4S)-3,4-dimethylhexane, which one is optically active? Briefly explain.arrow_forward
- Nonearrow_forwardNonearrow_forwardGiven the standard enthalpies of formation for the following substances, determine the reaction enthalpy for the following reaction. 4A (g) + 2B (g) → 2C (g) + 7D (g) AHrxn =?kJ Substance AH in kJ/mol A (g) - 20.42 B (g) + 32.18 C (g) - 72.51 D (g) - 17.87arrow_forward
- Determine ASran for Zn(s) + 2HCl(aq) = ZnCl2(aq) + H2(aq) given the following information: Standard Entropy Values of Various Substance Substance So (J/mol • K) 60.9 Zn(s) HCl(aq) 56.5 130.58 H2(g) Zn2+(aq) -106.5 55.10 CI (aq)arrow_forward3) Catalytic hydrogenation of the compound below produced the expected product. However, a byproduct with molecular formula C10H12O is also formed in small quantities. What is the by product?arrow_forwardWhat is the ΔHorxn of the reaction? NaOH(aq) + HCl(aq) → H2O(l) + NaCl(aq) ΔHorxn 1= ________ kJ/molarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


